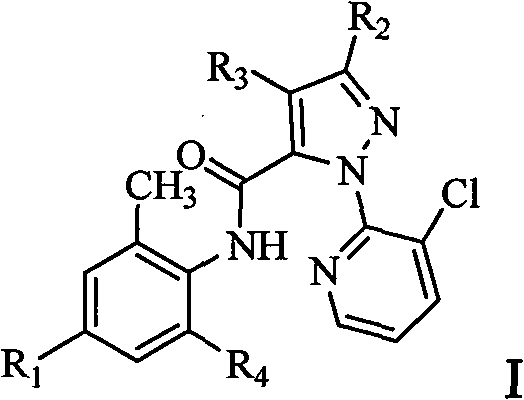
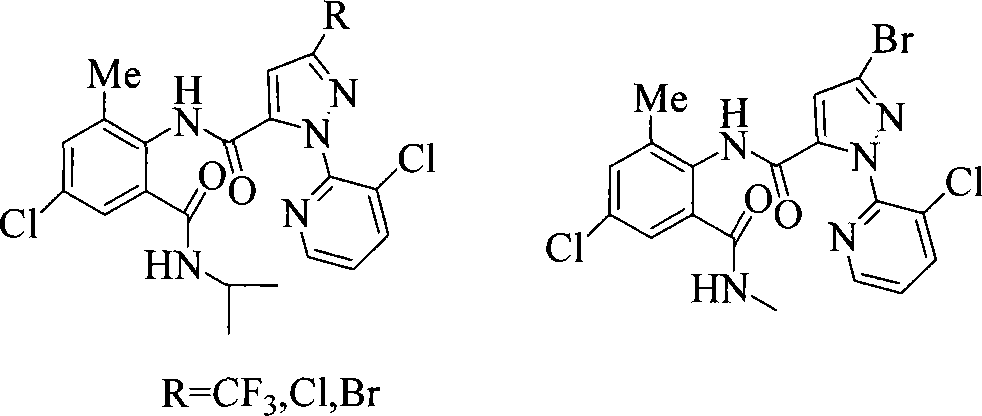
Anthranilic acid compound and use thereof
An anthranilic acid and compound technology, applied to N-oxides, anthranilic acid compounds, salts and compositions, as the application field of pesticides, can solve problems such as increased consumer spending and reduced productivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0121] Example 1: Preparation of compound 1-152
[0122]
[0123] Take 0.5 g of II-I in a 50 ml reaction flask, add 25 ml of tetrahydrofuran, add 1 g of HOCH with stirring 2 CH 2 N(CH 3 ) 2 , The temperature is raised and refluxed for 1 hour. After the reaction was monitored by TLC, after the solvent was removed under reduced pressure, the reaction flask was poured into 50 ml of saturated brine, extracted with 60 ml of ethyl acetate three times, dried, and dissolved, and column chromatography yielded 0.31 g of the product, which is the compound 1-152, the yield is 51.8%. Melting point 80-82°C.
[0124] NMR data ( 1 HNMR, 300MHz, internal standard TMS, solvent CDCl 3 )as follows:
[0125] Compound 1-152: δ ppm 2.18 (3H, s), 2.41 (6H, s), 2.79 (2H, t), 4.45 (2H, t), 7.19 (1H, s), 7.39 (2H, m), 7.80- 7.88 (2H, m), 8.45 (1H, q), 10.14 (1H, s).
example 2
[0126] Example 2: Preparation of compound 2-2
[0127]
[0128] Take 1 g of IV-1 in a 100 ml reaction flask, add 50 ml of dioxane and intermediate H 2 NC(CH 3 ) 2 CN, reflux for 3 hours. After the reaction was monitored by TLC, after the solvent was removed under reduced pressure, 50 ml of saturated brine was poured into the reaction flask, and the mixture was extracted three times with 90 ml of ethyl acetate, dried, and dissolved. The column chromatographed to obtain the III-1 intermediate 0.63. Grams.
[0129] Dissolve the prepared intermediate III-1 in 60 ml of tetrahydrofuran, add 1 ml of triethylamine, add 1.02 g of acid chloride dropwise with stirring, and react at room temperature for 4 hours. After the reaction is monitored by TLC, process the product and obtain 0.45 g of product by column chromatography. , Namely compound 2-2, yield 31%. The melting point is 170-172°C.
[0130] NMR data ( 1 HNMR, 300MHz, internal standard TMS, solvent CDCl 3 )as follows:
[0131] Compound ...
example 3
[0132] Example 3: Preparation of Compound 2-6
[0133]
[0134] Take 0.5 g of II-2 in a 50 ml reaction flask, add 25 ml of acetonitrile, add 0.8 g of H under stirring 2 NC(CH 3 ) 2 CN, heated and refluxed for 3 hours. After the reaction was monitored by TLC, after the solvent was removed under reduced pressure, 50 ml of saturated brine was poured into the reaction flask, and the mixture was extracted three times with 90 ml of ethyl acetate, dried and dissolved, and column chromatography yielded 0.27 g of the product, which is the compound 2-6, the yield is 45.3%. The melting point is 140-142°C.
[0135] NMR data ( 1 HNMR, 300MHz, internal standard TMS, solvent CDCl 3 )as follows:
[0136] Compound 2-6: δppm 1.95 (6H, s), 2.65 (3H, s), 6.85 (1H, s), 7.28 (1H, q), 7.87 (1H, s), 7.92 (1H, d), 8.30 ( 1H, q), 8.48 (1H, s).
[0137] Other compounds of general formula I can be prepared by the preparation method provided by the present invention.
[0138] Melting point and nuclear magnetic ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com