Punailuoer or delayed-release preparation of salt thereof and preparation method thereof
A delayed-release preparation, the technology of propranolol, which is applied in the field of delayed-release preparations of propranolol or its salts and its preparation, can solve problems such as the chronological rhythm of hypertension and angina pectoris without consideration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
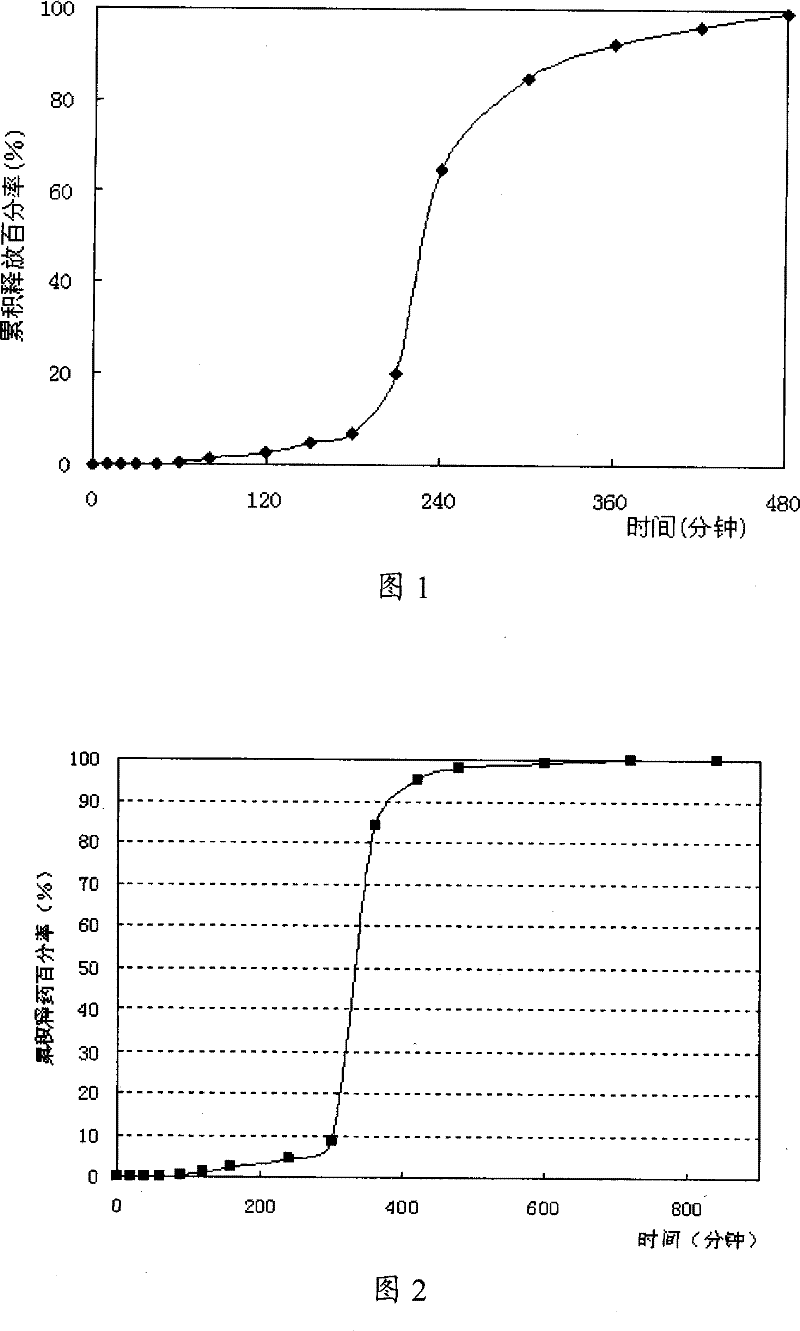
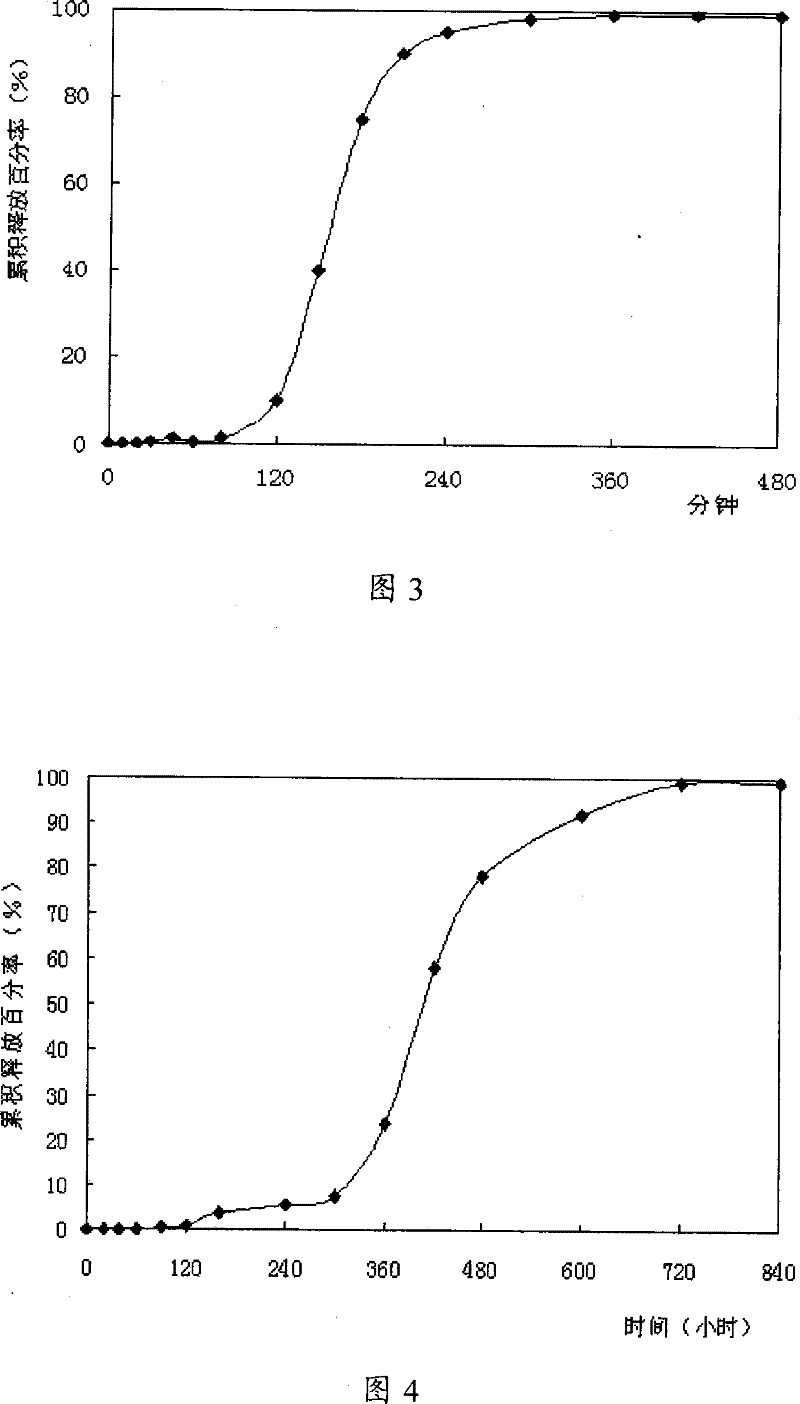
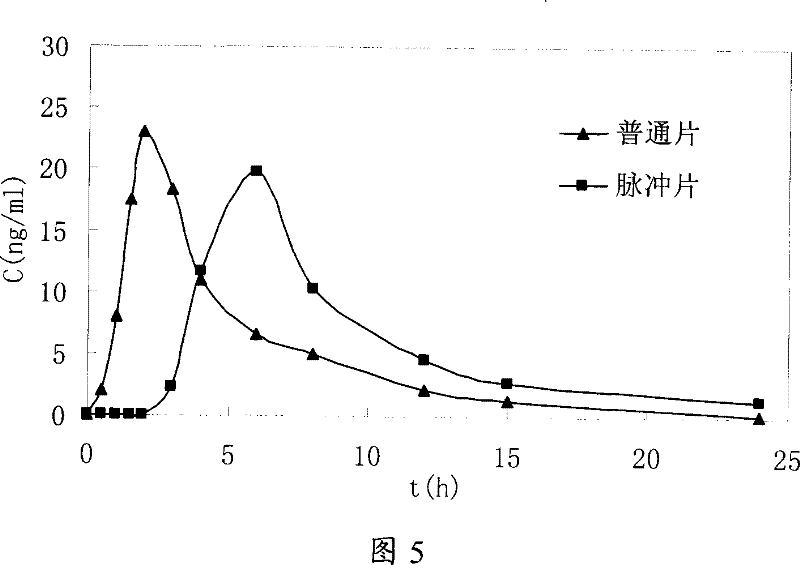
Image
Examples
Embodiment 1
[0058] Recipe 1:
[0059] Chip
[0060] Propranolol Hydrochloride 1.0g
[0061] Mannitol 2.5g
[0062] L-HPC 2.0g
[0063] Starch 0.5g
[0064] Magnesium stearate 0.1g
[0066] 100 pieces
[0067] Take propranolol hydrochloride, mannitol, L-HPC, and sodium chloride to pass through 80-mesh sieve, and mix evenly; take starch, add 10mL of water to boil, let it cool to make starch slurry, add mixed raw and auxiliary materials to prepare soft material , granulated through a 16-mesh sieve, dried at 65°C for 4 hours, granulated with a 18-mesh sieve, mixed with magnesium stearate. Use a 5.0mm shallow arc punch as a die, and press on a tablet press (TDP single-punch tablet press) to obtain a tablet core. The tablet core weighs 101mg and has a hardness of 3.5±0.5kg.
[0068] coating layer
[0069] Hydroxymethylcellulose 0.45g
[0070] Carnauba Wax 0.60g
[0071] Talc p...
Embodiment 2
[0078] Recipe 2:
[0079] Chip
[0080] Propranolol Hydrochloride 1.0g
[0081] Starch 3.5g
[0082] PVPP 0.5g
[0083] Povidone k29 0.1g
[0084] Magnesium stearate 0.1g
[0085] Potassium chloride 5.0g
[0086] 100 pieces
[0087] Take propranolol hydrochloride, starch, PVPP, and potassium chloride to pass through a 80-mesh sieve, mix evenly, dissolve povidone k29 in 10 mL of ethanol, add the mixed raw and auxiliary materials to make a soft material, and pass through a 16-mesh sieve to granulate , dried at 65°C for 3 hours, granulated with 18 mesh, mixed with magnesium stearate. Use a 5.0mm shallow arc punch as a die, and press a tablet machine to obtain tablet cores. The tablet core weighs 102mg and has a hardness of 4.5±0.5kg.
[0088] coating layer
[0089] Hydroxyethylcellulose 1.43g
[0090] Ethylcellulose 1.07g
[0091] Glyceryl Monostearate 0.71g
[0092] Triethyl citrate 0.36g
[0093...
Embodiment 3
[0096] Recipe 3:
[0097] Chip
[0098] Propranolol Hydrochloride 1.0g
[0099] Microcrystalline Cellulose PH101 3.4g
[0100] Mannitol 0.1g
[0101] CCNa 0.5g
[0102] Povidone k29 0.1g
[0103] Micronized silica gel 0.1g
[0104] Sorbitol 5.0g
[0105] 100 pieces
[0106] Take propranolol hydrochloride, microcrystalline cellulose PH101, CCNa, povidone k29, micropowder silica gel, sorbitol, and mannitol and pass through 80-mesh sieve respectively, mix evenly, and use the method of powder direct compression, with a thickness of 5.0 mm. The arc punching is a punching die, and the tablet core is obtained. The tablet core weighs 102mg and has a hardness of 3.5±0.5kg.
[0107] coating layer
[0108] Polyethylene glycol 0.41g
[0109] Beeswax 0.30g
[0110] Ethyl cellulose 0.01g
[0111] Glyceryl monostearate 0.20g
[0112] Triacetin 0.10g
[0113]Mix polyethylene glycol, beeswax, glycerin monostearate, glycero...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com