Anti-tumor DNA vaccine and preparation method thereof
A DNA vaccine and anti-tumor technology, applied in the field of medicine, can solve the problems of weak tumor targeting and DNA vaccine immunogenicity, and achieve the effect of improving immunogenicity, high safety, and strong targeting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0069] 1. Acquisition of mtHsp70 and HSV-tk genes
[0070] (1) Design primers:
[0071] According to the mRNA sequences of HSV-TK (genbank NO.J02224.) and Mycobacterium tuberculosis Hsp70 gene (genbank NO.BX842573), the specific primers for amplifying the full-length cDNA of HSV-TK and mtHsp70 genes were respectively designed by using the primer design software DNA club. The sequence is as follows:
[0072] HSV-TK primers:
[0073] Forward: 5 GCGCCTTGTAGA AGCGCG TA-3' (SEQ NO.1)
[0074] Reverse: 5' CCTTCCGGTATTGTCTCCCTTCC-3' (SEQ NO.2)
[0075] Both the upstream primer and the downstream primer introduce a restriction endonuclease Not I site (framed partial sequence);
[0076] Hsp 70 primer:
[0077] Forward: 5 CGCCTACATGGCTCGTGC-3' (SEQ NO.3)
[0078] R Reverse: 5 TCACTTGGCCTCCCGG-3' (SEQ NO.4)
[0079] Upstream and downstream primers introduce restriction endonuclease Xho I and EcoR I sites (framed partial sequences) respectively;
[0080] Submit the above-des...
example 2
[0147] 1. Acquisition of mt Hsp70 and HSV-tk genes: the same as Example 1.
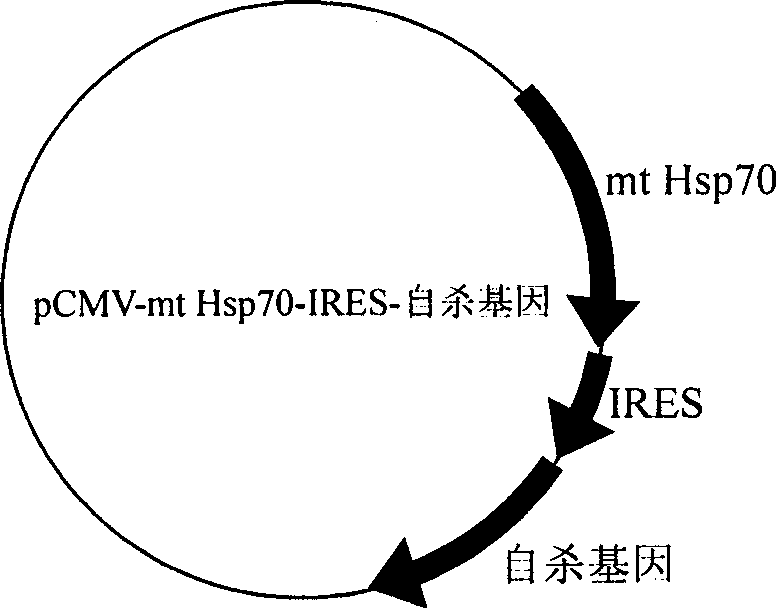
[0148] 2. Construction of pCMV-mt Hsp70-IRES-tk: same as Example 1.
[0149] 3. Preparation of the DNA vaccine of the present invention: transform pCMV-mtHsp70-IRES-tk into Lactobacillus, and the specific operation is the same as in Example 1.
example 3
[0151] 1. Acquisition of mt Hsp70 and HSV-tk genes: the same as Example 1.
[0152] 2. Construction of pCMV-mt Hsp70-IRES-tk: same as Example 1.
[0153] 3. Preparation of the DNA vaccine of the present invention: transform pCMV-mt Hsp70-IRES-tk into bifidobacteria, and the specific operation is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com