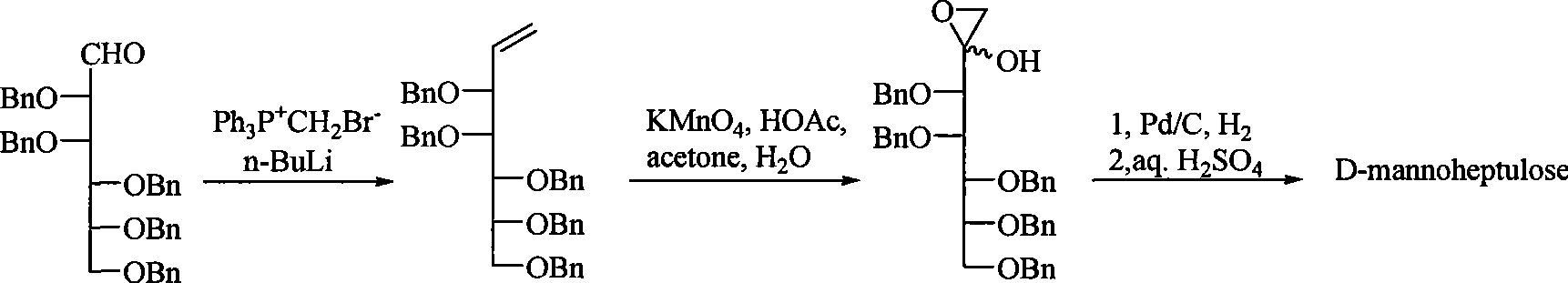Method for synthesizing D-mannoheptulose
A technology for mannoheptulose and mannoheptulose is applied in the field of synthesis of D-mannoheptulose, can solve the problems such as the complicated transformation method, and achieves the effects of low production cost, mild conditions and short process route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0017] The invention discloses a synthetic method of D-mannoheptulose, comprising the following steps:
[0018] Step 1: Preparation of 3,4,5,6,7-penta-O-benzylmannoheptosene,
[0019] in N 2 Under protection, add anhydrous toluene to triphenylmethylphosphine bromide, add n-butyl lithium in n-hexane solution dropwise to the system under ice-cooling, heat up, then add 2,3 , 4,5,6-penta-O-benzylmannose, after the reaction, add acetone and water to terminate the reaction, extract, wash, dry, concentrate, and obtain 3,4,5,6,7-penta by silica gel column chromatography -O-Benzylmannoheptosene;
[0020] In the above process, the Wittig reagent made of triphenylmethylphosphine bromide and n-butyllithium needs to be in excess of 2,3,4,5,6-penta-O-benzylmannose, and the dosage of the two is relative to The amount of 2, 3, 4, 5, 6-penta-O-benzylmannose should not be less than 2 times the molar equivalent; when preparing Wittig reagent, the reaction should be carried out in an ice bath ...
Embodiment 1
[0029] In the 100mL there-necked flask, add 12.8g (35.7mmol) triphenylmethylphosphine bromide, N 2 Add 35mL of anhydrous toluene under protection and stir well. Under an ice bath, add 23 mL of 1.6 mol / L BuLi n-hexane solution dropwise, and heat up to 35°C after 40 min. At this time, the reaction system appears dark yellow. Add 6.4 g (10.2 mmol) of 2,3 dissolved in 10 mL of anhydrous toluene dropwise, 4,5,6-penta-O-benzylmannose, 28°C reaction 2d. 10 mL of acetone and 10 mL of water were added to stop the reaction. Concentrate, 80mL CH 2 Cl 2 Dissolved, washed with 3 × 200mL water successively, Na 2 SO 4 Drying, filtration, concentration, and silica gel column chromatography (V (ethyl acetate): V (petroleum ether) = 1: 8 elution) gave 5.8 g of light yellow syrupy 3,4,5,6,7-penta- O-benzylmannoheptosene (88% yield).
[0030] Add 4.54g (7.2mmol) 3,4,5,6,7-penta-O-benzylmannoheptosene to a 250mL round bottom flask, add 70.3mL acetone, 13.9mL water, 3.2mL acetic acid to diss...
Embodiment 2
[0033] Add 12.6g (35.3mmol) triphenylmethylphosphine bromide in 100mL there-necked flask, N 2 Add 40mL of anhydrous toluene under protection and stir well. Under an ice bath, add 33 mL of 1.2 mol / L BuLi n-hexane solution dropwise, and heat up to 25°C after 1 hour. At this time, the reaction system appears dark yellow. Add 8.0 g (12.7 mmol) of 2,3 dissolved in 10 mL of anhydrous toluene dropwise, 4,5,6-penta-O-benzylmannose, react at room temperature for 36h. 10 mL of acetone and 10 mL of water were added to stop the reaction. Concentrate, 80mLCH 2 Cl 2 Dissolved, washed with 3 × 200mL water successively, Na 2 SO 4 Drying, filtration, concentration, and silica gel column chromatography (V (ethyl acetate): V (petroleum ether) = 1: 8 elution) gave 6.7 g of light yellow syrupy 3,4,5,6,7-penta- O-Benzylmannoheptosene (82% yield).
[0034] Add 5.60g (8.9mmol) 3,4,5,6,7-penta-O-benzylmannoheptosene to a 250mL round bottom flask, add 86.3mL acetone, 13.9mL water, 4.2mL acetic a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com