Preparation method of effective component of Hydrocotyle sibthorpioides and application
A technology of effective parts and coriander, applied in the field of extraction and application of traditional Chinese medicine, can solve the problems of no ideal drug, large toxic and side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] (1) The medicinal material of coriander is extracted 3 times with 8 times the weight of ethanol, the concentration of ethanol is 50%, and each time is 1 hour; the verification of the extraction process: take 2kg of the medicinal material, extract according to the best extraction process, and use the extraction method of HAS Obtaining rate is an investigation index, and the result is that the transfer rate of HAS is 87.2%, and the extraction rate of effective parts is 1.2% based on the total amount of HAS and HHAS;
[0019] (2) After the above-mentioned extract is decompressed and recovered ethanol, put it on the HPD400 type macroporous resin column, the concentration of the sample solution is 0.20mg (crude drug) / ml, and the ratio of the sample amount to the crude drug and resin is 1:4 (kg / L), eluted with 70% ethanol after 3 volumes of water elution;
[0020] (3) Take the purified effective part, use silica gel column chromatography, elute with CHCl3:MeOH:H20=20:3:1 (up...
Embodiment 2
[0022] (1) Coriander Coriander medicinal material is extracted 2 times with 6 times the weight of ethanol, the concentration of ethanol is 60%, each time for 1 hour;
[0023] (2) After the above-mentioned extract is decompressed and recovered ethanol, put it on the HPD400 type macroporous resin column, the concentration of the sample solution is 0.25mg (crude drug) / ml, and the ratio of the sample amount to the crude drug and resin is 1:4 (kg / L), eluted with 50% ethanol after 3 volumes of water elution;
[0024] (3) Take the purified effective part, use silica gel column chromatography, elute with CHCl3:MeOH:H2O=20:3:1 (upper layer)-CHCl3:MeOH:H2O=10:3:1 (upper layer), collect CHCl3:MeOH:H2O=10:3:1 eluate, recover the solvent under reduced pressure, and recrystallize multiple times with methanol to obtain HAS with a yield of 0.52% (HSS: crude drug).
Embodiment 3
[0026] (1) Coriander Coriander medicinal material is extracted once with 10 times the weight of ethanol, and the concentration of ethanol is 70%;
[0027] (2) Go up NKA-9, D101, AB-8 or HPD100 macroporous resin column after decompression recovery ethanol of above-mentioned extract solution, the concentration of sample solution is 0.25mg (crude drug) / ml, 0.30mg (crude drug) / ml ml, 0.15mg (crude drug) / ml sample amount equivalent to the ratio of crude drug to resin is 1:4 (kg / L), eluted with 3 volumes of water and then eluted with 60% ethanol;
[0028] (3) Take the purified effective part, use silica gel column chromatography, elute with CHCl3:MeOH:H2O=20:3:1 (upper layer)-CHCl3:MeOH:H2O=10:3:1 (upper layer), collect CHCl3:MeOH:H2O=10:3:1 eluate, recover the solvent under reduced pressure, and recrystallize multiple times with methanol to obtain HAS with a yield of 0.49% (HAS: crude drug).
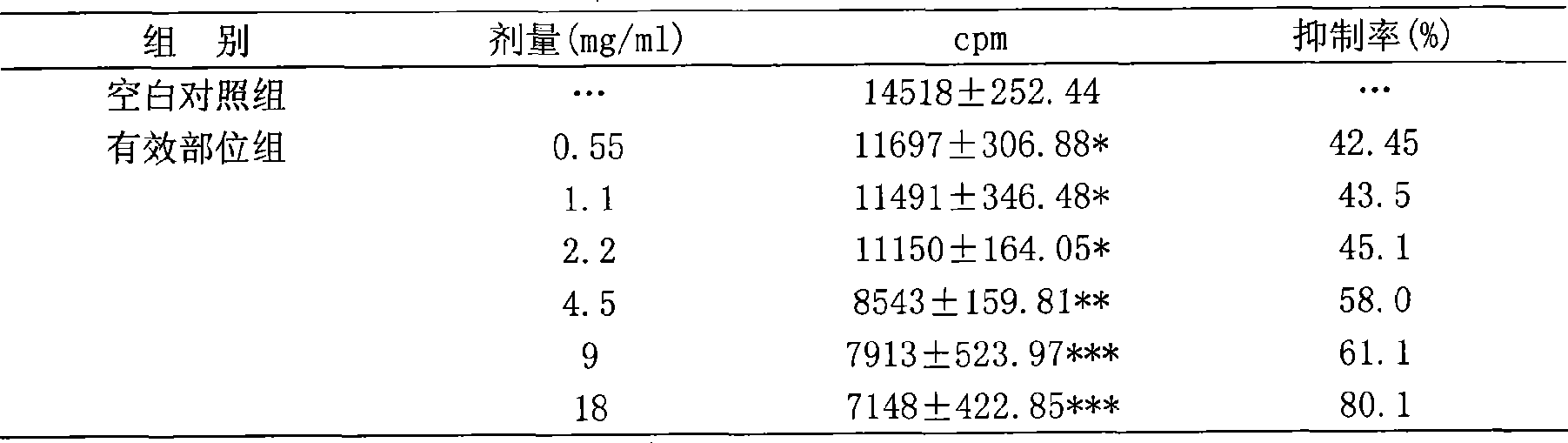
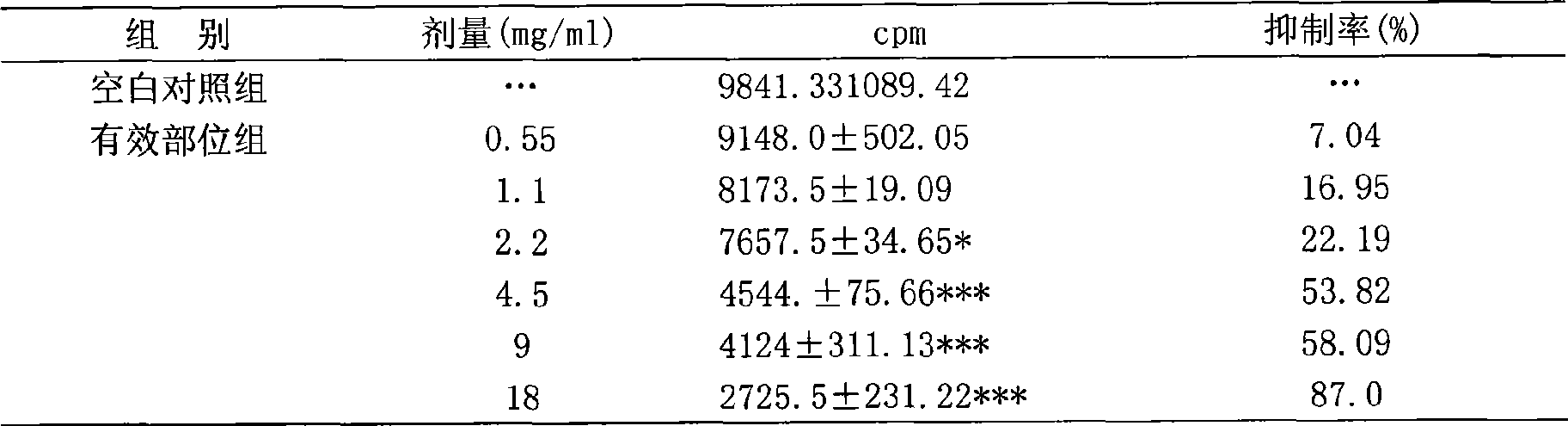
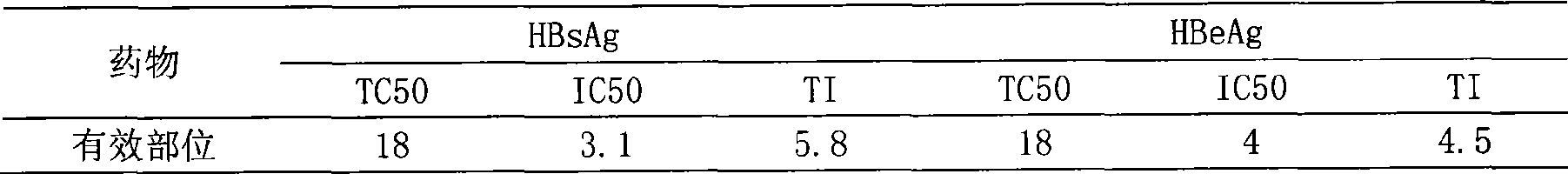
[0029] In vitro Inhibition Test of One Effective Fraction on HepG2 Cells Transfected by ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com