Triazoles compounds with antimicrobial activity and preparation method and pharmaceutical use thereof
A compound, triazole technology, applied in the field of new triazole compounds, can solve the complicated treatment of deep fungal infection and other problems, and achieve the effect of low cost, easy to obtain raw materials, and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
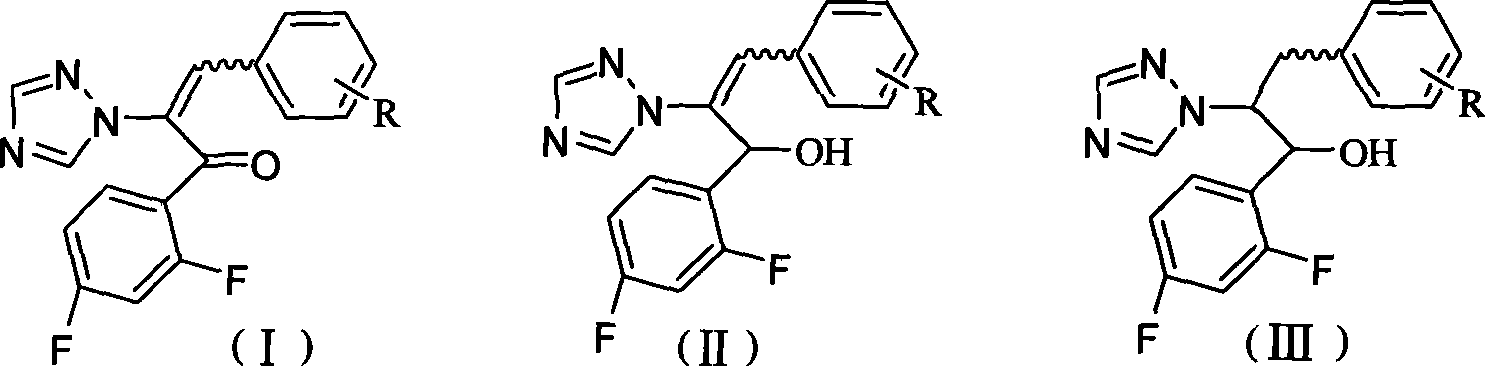
[0029] Example 1: (E)-3-(4-chlorophenyl)-1-(2,4-difluorophenyl)-2-(1H-1,2,4-triazol-1-yl)- 2-propen-1-one (compound 1a for short) and (Z)-3-(4-chlorophenyl)-1-(2,4-difluorophenyl)-2-(1H-1,2,4 Preparation of -triazol-1-yl)-2-propene-1-one (compound 1b for short)
[0030] Add 2.23g (0.01mol) 1-(2,4-difluorophenyl)-2-(1H-1,2,4-triazol-1-yl)ethanone, 4-chloro 1.41g (0.01mol) of benzaldehyde, 0.5mL of hexahydropyridine and 40mL of toluene, install a water separator, reflux condenser, and heat to reflux for 6h under the protection of nitrogen. After cooling slightly, it was washed with saturated brine, and the organic phase was separated. The aqueous phase was extracted with chloroform (20 mL×3), the organic phases were combined, dried over anhydrous magnesium sulfate, concentrated and purified by silica gel column chromatography to obtain 2.61 g of a light yellow solid, namely compound 1a, with a yield of 75.4%; Melting point: 102-104°C; at the same time, 0.45 g of another light...
Embodiment 2
[0031] Example 2: Hydroxylation of (E)-1-[3-(3,4-dichlorophenyl)-1-(2,4-difluorophenyl)-2-(1H-1,2,4- Preparation of triazol-1-yl)allyl]piperidinium (referred to as compound 2)
[0032] Compound 2 was synthesized according to the preparation method of compound 1, and the starting material was 2.23 g of 1-(2,4-difluorophenyl)-2-(1H-1,2,4-triazol-1-yl)ethanone (0.01mol), 3,4-dichlorobenzaldehyde 1.75g (0.01mol), the product obtained is light yellow solid 2.52g, is compound 2, productive rate: 65.8%; m.p.141~143 ℃; UV-vis ( CH 3 OH)λ max : 205, 296nm; IR (KBr): 3121.3 (Ar-H, =C-H), 1603.8, 1498.6, 1427.3 (aromatic ring skeleton, C = C), 842.8 (=C-H) cm -1 ; 1 HNMR (300MHz, CDCl 3 )δ: 8.06(d, 1H, triazole3-H), 8.04(d, 1H, triazole5-H), 6.60~7.40(m, 7H, Ar-H, C=CH), 2.88~2.90(t, 4H, piperidine 2,6-H)1.50~1.72(m,6H,piperidine 3,4,5-H)ppm; 13 C NMR (300MHz, CDCl 3 )δ: 192.1 (C=N + -), 167.1, 163.7, 154.4, 152.5, 145.2, 136.9, 135.4, 133.9, 132.5, 132.3., 131.9, 131.1, 128.5...
Embodiment 3
[0033] Example 3: (E)-3-(4-methoxyphenyl)-1-(2,4-difluorophenyl)-2-(1H-1,2,4-triazol-1-yl )-2-propen-1-ketone (abbreviation compound 3) preparation
[0034] Compound 3 was synthesized according to the preparation method of compound 1, the starting material was 2.23 g of 1-(2,4-difluorophenyl)-2-(1H-1,2,4-triazol-1-yl)ethanone (0.01mol), p-methoxybenzaldehyde 1.36g (0.01mol), the product obtained is light yellow solid 2.60g, which is compound 3, yield: 76.8%; m.p.144~145°C; UV-vis (CH 3 OH)λ max : 343nm; IR (KBr): 3055.8 (Ar-H, =C-H), 2966.1, 2839.5 (CH 3 ), 1660.5 (C=O), 1606.1, 1507.3, 1460.2 (aromatic ring skeleton, C=C), 833.4 (=C-H) cm -1 ; 1 H NMR (300MHz, CDCl 3 )δ: 8.10 (d, 2H, triazole-H), 6.74~7.57 (m, 8H, Ar-H, C=CH), 3.73 (s, 3H, OCH 3 ) ppm; 13 C NMR (300MHz, CDCl 3 )δ: 187.1 (C=O), 163.3, 162.8, 158.7, 153.0, 145.3, 142.8, 133.3, 132.9, 131.9, 131.0, 123.3, 122.4, 114.8, 114.1, 112.4, 104.8 (Ar-C, triazole-C, C=CH), 55.5 (OCH 3 )ppm; MS (m / z): 342[M] + ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com