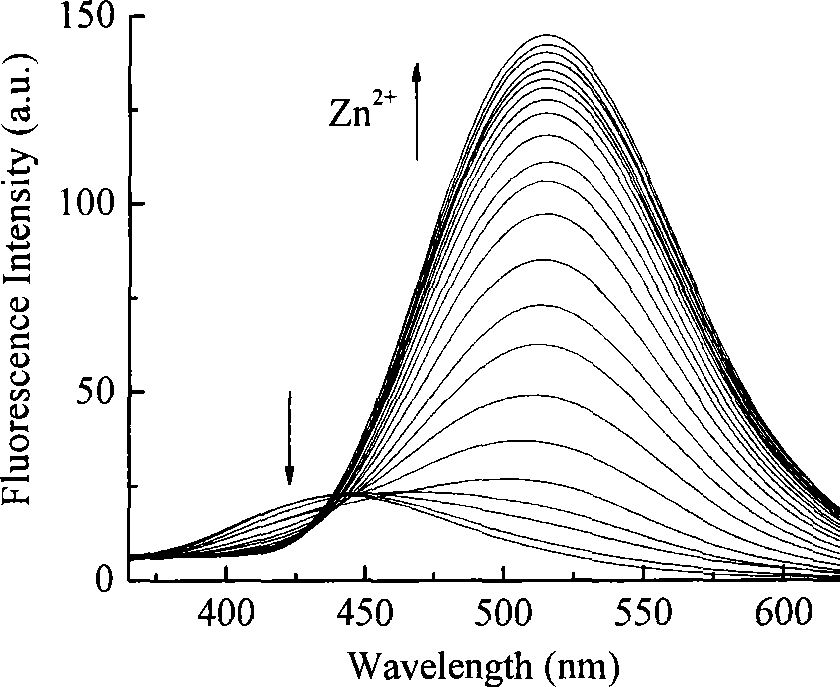
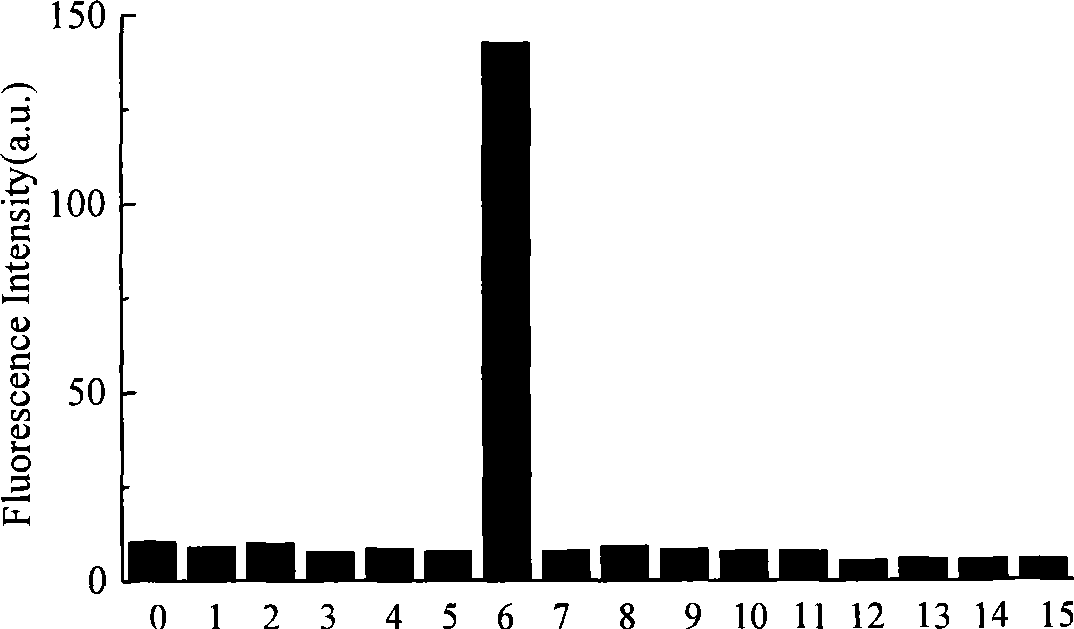Synthesis of N-acyl-8-amino quinoline derivatives and use thereof as fluorescent molecular probe
The technology of aminoquinoline and acyl group is applied in the synthesis of N-acyl-8-aminoquinoline derivatives and its application field as a fluorescent molecular probe, which can solve the problems of poor water solubility, short emission wavelength, weak fluorescence and the like, To achieve the effect of enhanced fluorescence intensity, easy availability of raw materials and simple synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026]
[0027] Dissolve 288mg (2.0mmol) of 8-aminoquinoline and 222mg (2.8mmol) of pyridine in 10mL of chloroform, add 5mL of chloroform solution containing 461mg (2.4mmol) of chloroacetyl chloride dropwise within 1 hour under ice cooling, Stir at room temperature for 2h to stop the reaction. The reaction liquid was spin-dried and purified by column chromatography, the yield was 79.9%, mp: 131.6-132.6°C. Further 80mg (0.362mmol) 8-chloroacetyl quinoline, 381mg (3.62mmol) aminoethoxyethanol, 468mg (3.62mmol) DIPEA and 20mgKI were added to 30mL acetonitrile, N 2 Under the protection of reflux for 10h, stop the reaction. The reaction solution was spin-dried and purified by column chromatography with a yield of 83.8%. 1 H-NMR (400MHz, CDCl 3 ): 11.24(CONH, s, 1H), 8.86(d, J=5.6Hz, 1H), 8.81(d, J=8.8Hz, 1H), 8.16(d, J=9.6Hz, 1H), 7.53(m , 2H), 7.46(m, 1H), 3.74(OCH 2 CH 2 -OH, m, 4H), 3.59 (COCH 2 , NHCH 2 -CH 2 , m, 4H), 2.99 (CH 2 NH-CH 2 , t, J=4.8Hz, 2H).
Embodiment 2
[0029]
[0030] Operation method is referring to embodiment 1. Yield: 79.4%, mp: 112.4°C. 1H-NMR (400MHz, DMSO): 8.95(d, J=8.0Hz, 1H), 8.69(d, J=8.8Hz, 1H), 8.43(d, J=9.6Hz, 1H), 7.66(m, 3H ), 3.67 (CH 2 -OH, t, J=5.2Hz, 2H), 3.48 (COCH 2 , s, 2H), 3.14 (NH-CH 2 CH 2 OH, t, J=6.4Hz, 2H), 3.09 (NHCH 2 -CH 2 NH, t, J=5.2Hz, 2H), 2.92 (COCH 2 NH-CH 2 , t, J=6.6Hz, 2H).
Embodiment 3
[0032]
[0033] Operation method is referring to embodiment 1. Yield: 62.7%, mp: 183.0-184.2°C. 1 H-NMR (400MHz, DMSO): 8.97 (d, J = 5.2Hz, 1H), 8.63 (d, J = 7.6Hz, 1H), 8.45 (d, J = 9.6Hz, 1H), 8.76 (d, J =8.0Hz, 2H), 7.66(m, 2H), 4.12(COCH 2 , s, 2H), 3.69 (CH 2 -OH, t, J=5.4Hz, 2H), 3.05(CH 2 NH-CH 2 , t, J=5.4Hz, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com