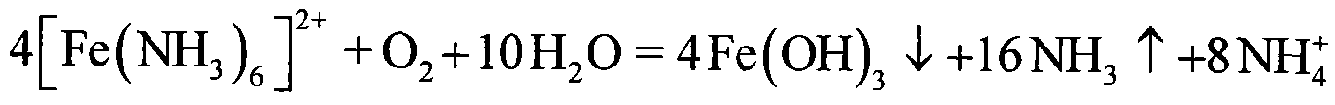Method for extracting nickel oxide from lateritic nickel
A technology for laterite nickel ore and nickel oxide is applied in the field of extracting nickel oxide from laterite nickel ore and processing laterite nickel ore, which can solve the problems of high microbial culture cost, long production cycle, influence on technological process and the like, and achieves low cost and simple equipment. , the effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
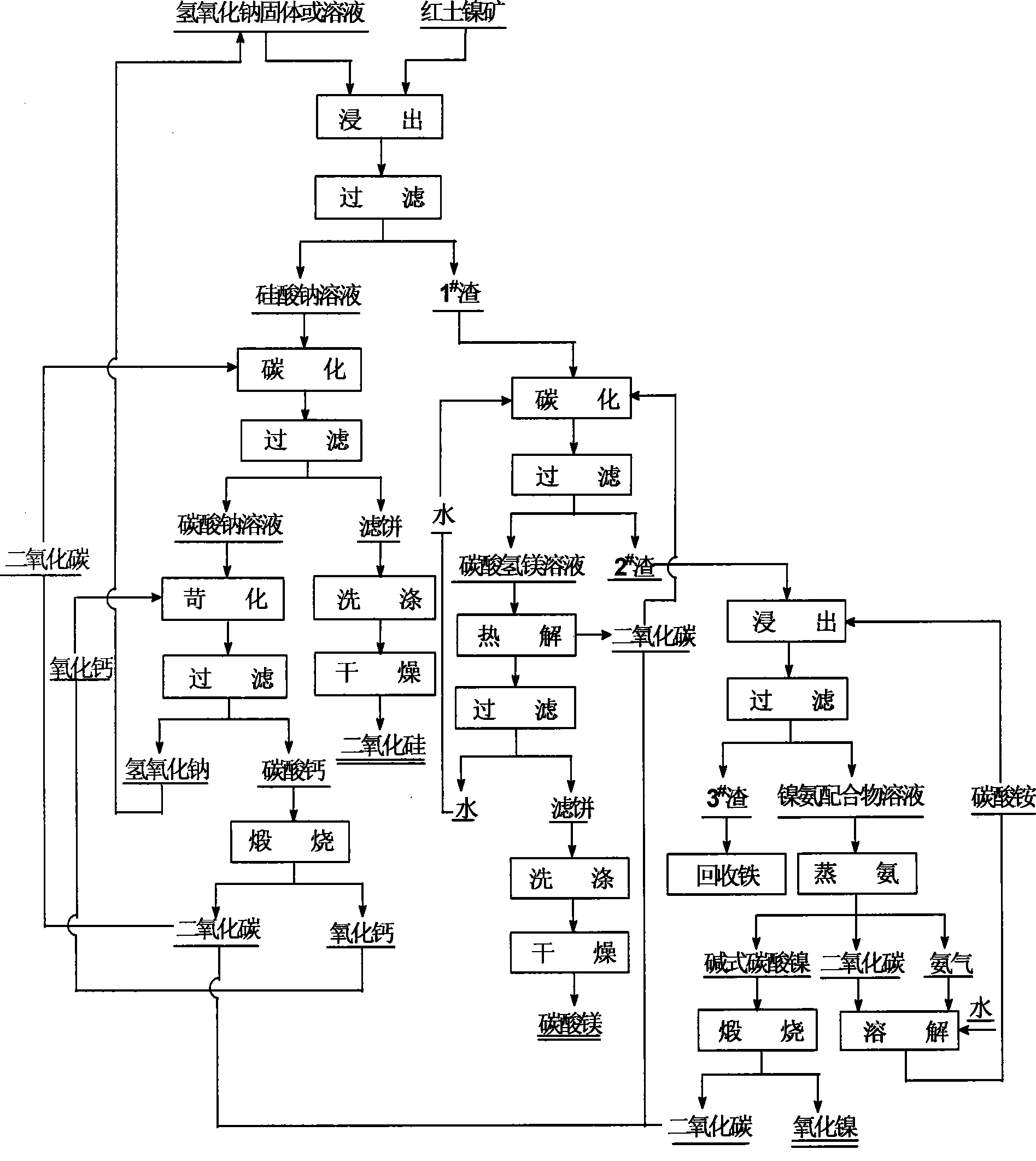
Image
Examples
Embodiment 1
[0037] The composition of the laterite nickel ore used is: NiO 0.91%, SiO 2 38.74%, MgO 22.53%, Fe 2 o 3 19.82%, Al 2 o 3 4.65%, CaO 0.62%, Cr 2 o 3 0.55%, other impurities 0.78%, loss on ignition 11.4%.
[0038] Mix the laterite nickel ore that is ground to less than 80 μm and solid sodium hydroxide at a mass ratio of 1:3, react at 500°C with stirring for 30 minutes, then stop heating, and when the temperature is lowered to 90°C, add 4 times the volume of water , boiled and dissolved at 85°C for 40 minutes, filtered, the filtrate was sodium silicate solution, and the filter cake was 1 # scum.
[0039] The sodium silicate solution was heated to 80°C, and under stirring conditions, carbon dioxide gas was introduced at a flow rate of 100ml / min until the pH value of the solution dropped to 9, and then filtered to obtain sodium carbonate solution and filter cake. After the filter cake is washed to neutral, it is dried at 60° C. for 10 hours to obtain a silica powder wit...
Embodiment 2
[0044] The composition of the laterite nickel ore used is: NiO 1.03%, SiO 2 40.57%, MgO 20.31%, Fe 2 o 3 18.66%, Al 2 o 3 3.87%, CaO 0.68%, Cr 2 o 3 0.52%, other impurities 0.86%, loss on ignition 13.65%.
[0045] Mix the laterite nickel ore that is ground to less than 80 μm and the sodium hydroxide solution with a concentration of 75% at a mass volume ratio (g:ml) of 1:5, and react for 1.5 hours at 225°C with stirring, and then stop heating. Cool down to 80°C, add 5 times the volume of water to dilute, continue leaching at 85°C for 20 minutes, filter and separate, the filtrate is sodium silicate solution, and the filter cake is 1 # scum.
[0046] The sodium silicate solution was heated to 85°C, and under stirring conditions, carbon dioxide gas was introduced at a flow rate of 150ml / min until the pH value of the solution dropped to 8.5, and filtered to obtain sodium carbonate solution and filter cake. After the filter cake is washed to neutral, it is dried at 70° C....
Embodiment 3
[0051] The composition of the laterite nickel ore used is: NiO 1.21%, SiO 2 36.88%, MgO 24.63%, Fe 2 o 3 21.95%, Al 2 o 3 5.05%, CaO 0.57%, Cr 2 o 3 0.61%, other impurities 0.46%, loss on ignition 12.05%.
[0052] Mix the laterite nickel ore that is ground to less than 80 μm and the sodium hydroxide solution with a concentration of 85% at a mass volume ratio (g:ml) of 1:4, and react for 1 hour at 250°C with stirring, then stop heating and cool down When the temperature reaches 90°C, add 3 times the volume of water to dilute, continue leaching at 80°C for 30 minutes, then filter, the filtrate is sodium silicate solution, and the filter cake is 1 # scum.
[0053] Heat the sodium silicate solution to 90°C, under the condition of strong stirring, pass carbon dioxide gas at a flow rate of 100ml / min until the pH value of the solution drops to 9, filter to obtain sodium carbonate solution and filter cake. After the filter cake is washed to neutral, it is dried at 70°C for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com