Method for simultaneously determining of concentration multi anesthesia medicament in blood plasma
A technology of local anesthesia and drug concentration, applied in the field of medical testing, to achieve the effect of less sampling, strong selectivity, and improved detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Chromatographic conditions
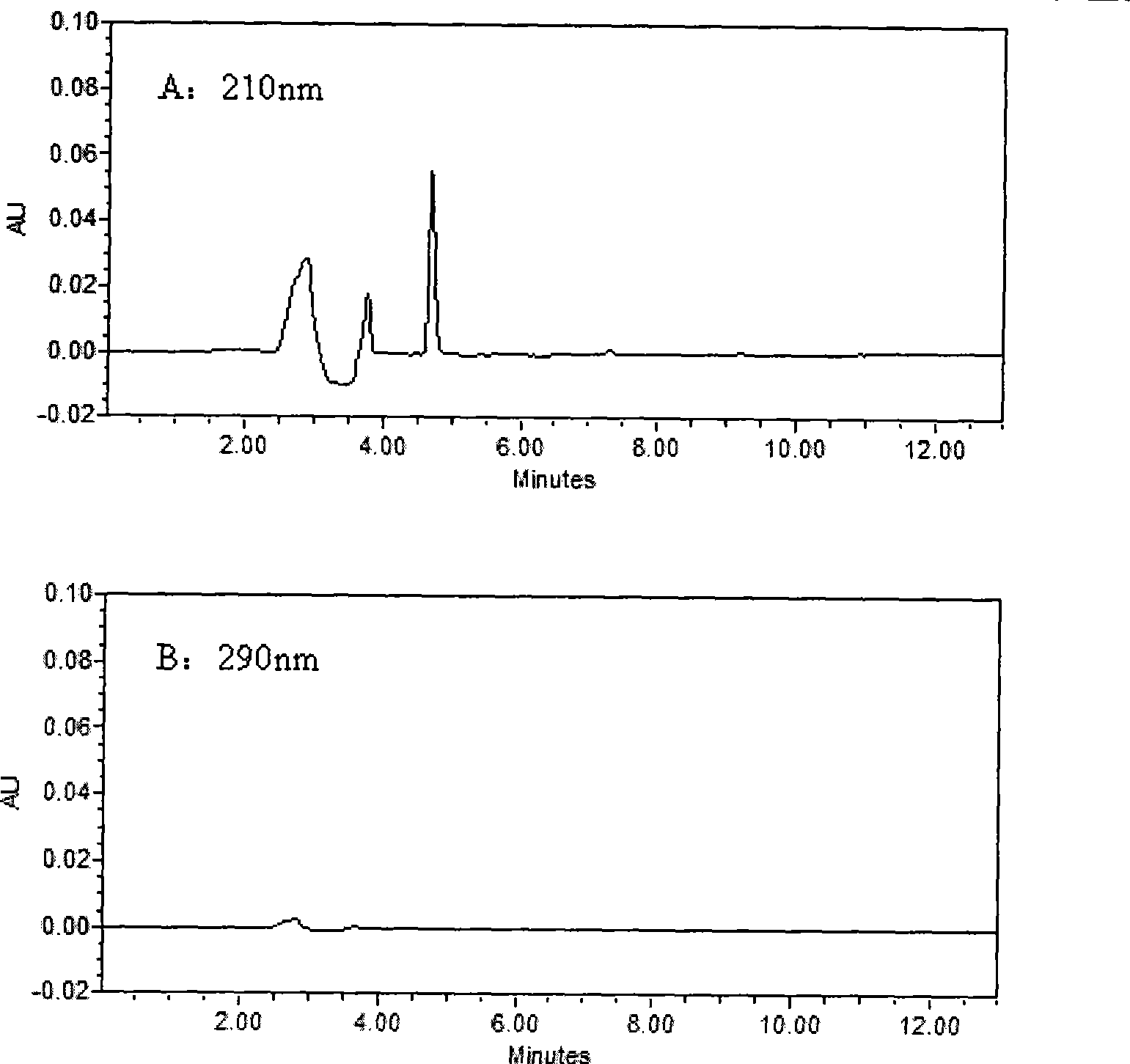
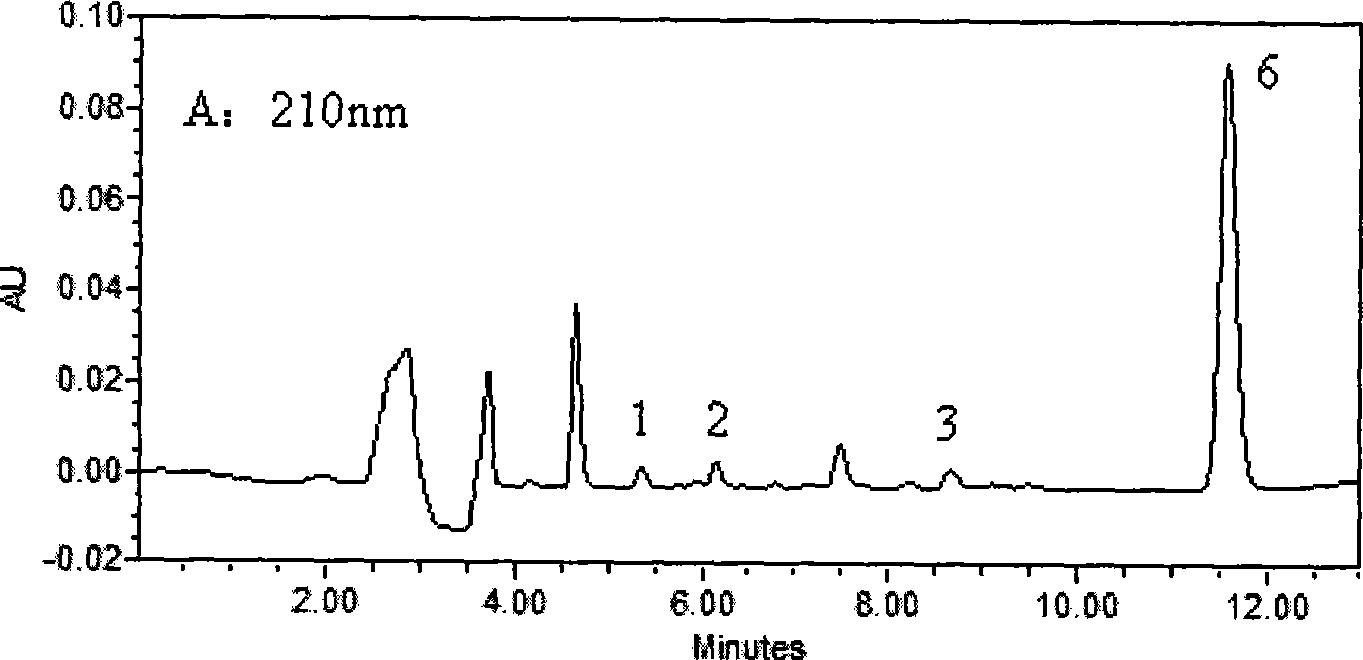
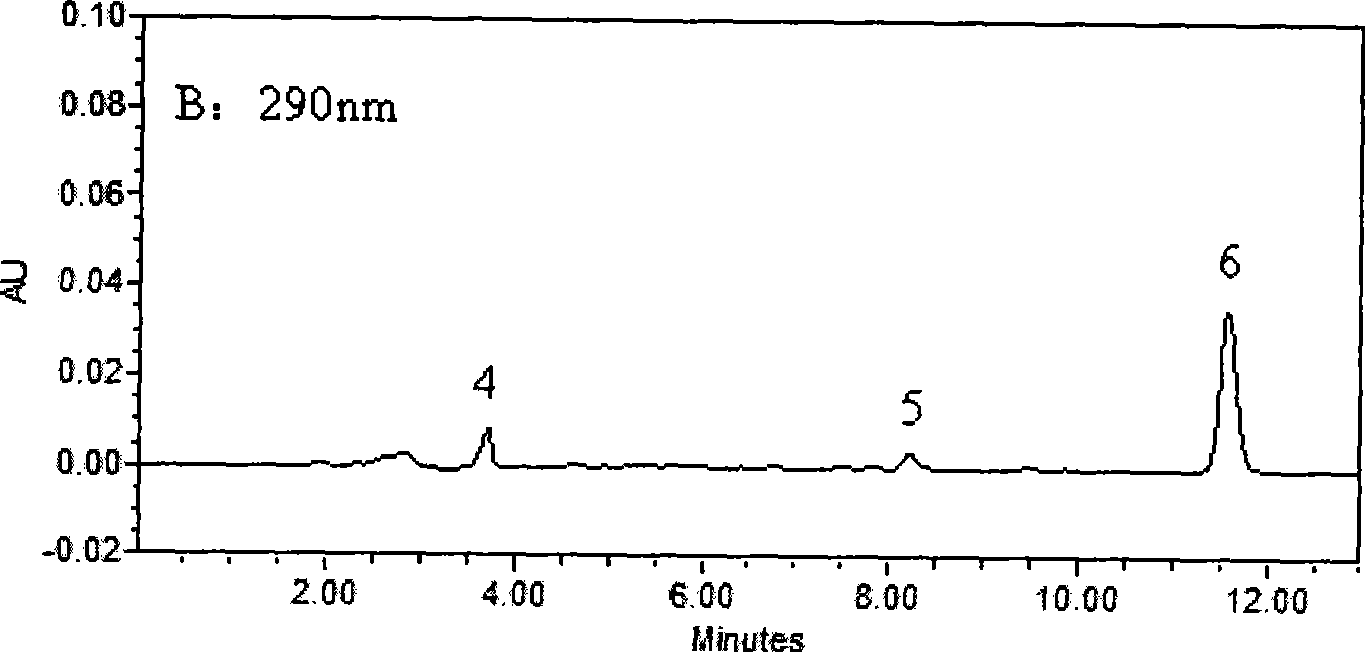
[0030] Waters2690HPLC system, Waters2487 dual-wavelength UV detector, Millennium32 chromatographic workstation (Version4.0); chromatographic column: KromasilC 18 (250mm×4.6mm, 5μm); Column temperature: 40°C; Mobile phase: 30mmol / L potassium dihydrogen phosphate aqueous solution (containing 0.14% triethylamine solution, phosphoric acid solution to adjust pH=4.8): acetonitrile (61:39, V / V); flow rate 1.0mL min -1 ; The concentration of LID, ROP and BUP was measured at 210nm, and the concentration of PRO and TET was measured at 290nm.
[0031] Plasma sample pretreatment
[0032] Take 0.5mL blood sample and add 50μl of 0.5mg / mL neostigmine solution, 100μl of 5μg / mL internal standard solution and 100μL of 0.5mol / L KaOH solution, vortex for 10Sec, mix well, add 3mL of diethyl ether, all the above operations are at 3℃ Carry out under the following ice bath conditions; vortex for 2min, centrifuge at 2500g×8min, take 2.5mL of supernatant in anoth...
Embodiment 2
[0044] Chromatographic conditions
[0045] Waters2690HPLC system, Waters2487 dual-wavelength ultraviolet detector, Millennium32 chromatographic workstation (Version4.0); Chromatographic column: Kromasil C 18 (250mm×4.6mm, 5μm); Column temperature: 40°C; Mobile phase: 30mmol / L potassium dihydrogen phosphate aqueous solution (containing 0.18% triethylamine solution, phosphoric acid solution to adjust pH=5.1): acetonitrile (65:35, V / V); flow rate 1.0mL min -1 ; The concentration of LID, ROP and BUP was measured at 210nm, and the concentration of PRO and TET was measured at 290nm.
[0046] Plasma sample pretreatment
[0047] Take 0.5mL blood sample and add 50μl of 2mg / mL neostigmine solution, 100μl of 5μg / mL internal standard solution and 100μL of 2mol / L NaOH solution, vortex for 8Sec, mix well, add 3mL of diethyl ether, the above operations are all under 3°C on ice Carry out under bath conditions; vortex for 2min, centrifuge at 2500g×10min, take 2.5mL supernatant in another t...
Embodiment 3
[0058] Chromatographic conditions
[0059] Waters2690HPLC system, Waters2487 dual-wavelength ultraviolet detector, Millennium32 chromatographic workstation (Version4.0); Chromatographic column: Kromasil C 18 (250mm×4.6mm, 5μm); Column temperature: 40°C; Mobile phase: 30mmol / L potassium dihydrogen phosphate aqueous solution (containing 0.16% triethylamine solution, phosphoric acid solution to adjust pH=4.9): acetonitrile (63:37, V / V); flow rate 1.0mL min -1 ; The concentration of LID, ROP and BUP was measured at 210nm, and the concentration of PRO and TET was measured at 290nm.
[0060] Plasma sample pretreatment
[0061] Take 0.5mL blood sample and add 50μl of 1mg / mL neostigmine solution, 100μl of 5μg / mL internal standard solution and 100μL of 1mol / L NaOH solution, vortex for 12Sec, mix well, add 3mL of diethyl ether, the above operations are all under 3°C on ice Under bath conditions; vortex for 2min, centrifuge at 3000g×9min, take 2.5mL of supernatant in another test tub...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com