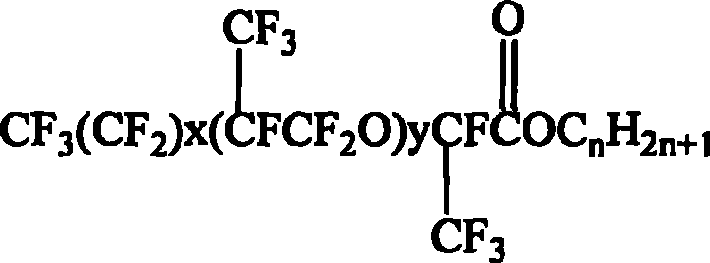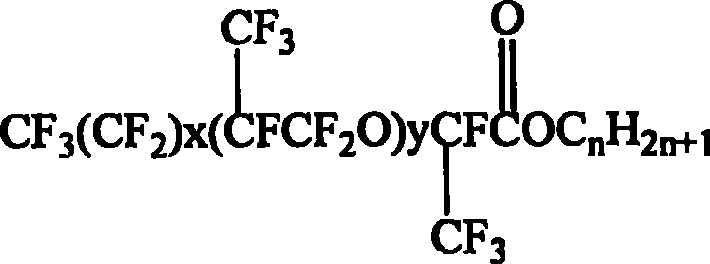Synthesis of hexafluoropropylene oxide oligomer type fluorocarbon surfactant and use thereof
A technology of fluorocarbon surfactants and active agents, applied in chemical/physical processes, dissolution, chemical instruments and methods, etc., can solve the problems of small number of fluorocarbon surfactants, poor product performance, environmental hazards, etc., and achieve detergency Odorless, reduced surface tension, strong defoaming effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Into a 5L stainless steel autoclave, 600ml of diethylene glycol dimethyl ether, 120ml of tetramethylethylenediamine, 10g of cesium fluoride, 4ml of triethylamine were passed into the 5L stainless steel autoclave, the reaction temperature was set to 20℃, and the hexafluoro ring was continuously passed in. Oxypropane 200g, reaction time 3h. After the reaction is over, continue to stir for 60 minutes, stand for 60 minutes, and discharge. After standing for layering and liquid separation, the lower layer was taken out and analyzed by gas chromatography for composition and distillation to obtain perfluoro-2,5-dimethyl-3,6-dioxononanoyl fluoride.
[0027] Under the action of acid binding agent, 0.21 mol of perfluoro-2,5-dimethyl-3,6-dioxononanoyl fluoride was slowly added dropwise to a three-necked flask containing 0.20 mol of n-octanol, and stirred at 40°C Reflux for 6.5h, wash the resultant product with distilled water to PH=6, then let it stand overnight with anhydrous magnesi...
Embodiment 2
[0029] Into a 5L stainless steel autoclave, 600ml of diethylene glycol dimethyl ether, 120ml of tetramethylethylenediamine, 10g of cesium fluoride, 4ml of triethylamine were passed into the 5L stainless steel autoclave, the reaction temperature was set to 20℃, and the hexafluoro ring was continuously passed in. Oxypropane 200g, reaction time 3h. After the reaction is over, continue to stir for 60 minutes, stand for 60 minutes, and discharge. After standing for layering and liquid separation, the lower layer was taken out and analyzed by gas chromatography for composition and distillation to obtain perfluoro-2,5-dimethyl-3,6-dioxononanoyl fluoride.
[0030] Under the action of acid binding agent, 0.21mol perfluoro-2,5-dimethyl-3,6-dioxononanoyl fluoride was slowly added dropwise to a three-necked flask containing 0.20mol n-dodecanol, at 50℃ After stirring and refluxing for 7 hours, the obtained product was washed with distilled water to PH=6, and then left to stand overnight with a...
Embodiment 3
[0032] Into a 5L stainless steel autoclave, 600ml of diethylene glycol dimethyl ether, 120ml of tetramethylethylenediamine, 10g of cesium fluoride, 4ml of triethylamine were passed into the 5L stainless steel autoclave, the reaction temperature was set to 20℃, and the hexafluoro ring was continuously passed in. Oxypropane 200g, reaction time 3h. After the reaction is over, continue to stir for 60 minutes, stand for 60 minutes, and discharge. After standing for layering and liquid separation, the lower layer was taken out and analyzed by gas chromatography for composition and distillation to obtain perfluoro-2,5-dimethyl-3,6-dioxononanoyl fluoride.
[0033] Under the action of acid binding agent, 0.21mol perfluoro-2,5-dimethyl-3,6-dioxononanoyl fluoride was slowly added dropwise to a three-necked flask containing 0.20mol n-hexadecanol at 50℃ After stirring and refluxing for 8 hours, the obtained product was washed with distilled water to PH=6, then stood overnight with anhydrous ma...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface tension | aaaaa | aaaaa |
| surface tension | aaaaa | aaaaa |
| surface tension | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com