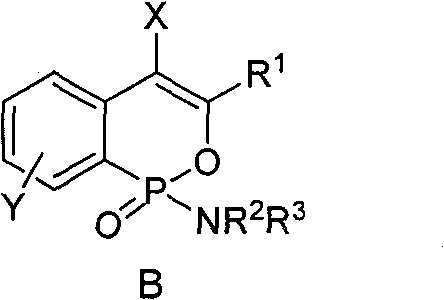
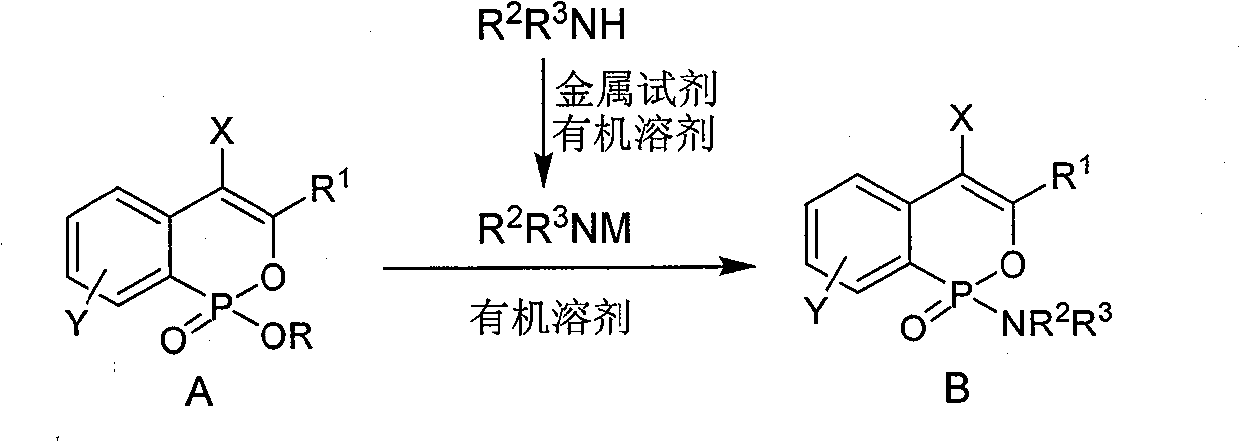
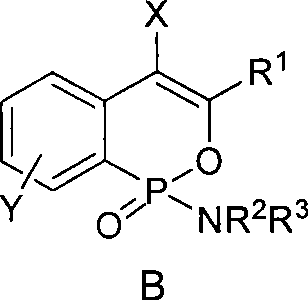
Phosphine isocoumarin amide, synthesis and uses thereof
An isocoumarin amide and reaction technology, which is applied in the field of phosphinocoumarin amide compounds and their synthesis, can solve the problems affecting the biological activity research of such compounds, and achieve a simple and efficient synthesis method, good cholesterol esterase, inhibit cholesterol The effect of esterase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026]
[0027] Typical operation of DIBAL-H as a metal reagent: under nitrogen protection, add PhCH to a 50mL dry round bottom flask 2 NH 2 (0.96mL, 8.8mmol), toluene (5.0mL), under an ice-water bath, slowly add 1M DIBAL-H toluene solution (8.80mL, 8.8mmol) dropwise, and continue to react at 0°C for 1 hour after the drop.
[0028] Slowly add 3-phenyl-7-methoxyphosphine isocoumarin ethyl ester (475mg, 1.5mmol) in toluene (5.0mL) dropwise to the above reaction solution, after the dropwise addition, naturally rise to room temperature and react for 12 hours, The reaction solution was cooled to 0°C, washed with water (10mL), 1M KHSO 4 Wash with aqueous solution, extract with ethyl acetate (3×10 mL), dry over anhydrous sodium sulfate, filter, evaporate the solvent under reduced pressure, and obtain 370 mg of white solid by column chromatography (P:E=6:1-1:1) of the residue. For the corresponding phosphine isocoumarinamide, the yield is 65%. 1 H NMR: δ7.72(d, J=7.5Hz, 2H), 7.2...
Embodiment 2
[0030]
[0031] Typical operation of n-butyllithium (n-BuLi) as a metal reagent: under nitrogen protection, in a 50mL dry round bottom flask, add cyclohexylamine (0.27mL, 2.5mmol), anhydrous 1,4-dioxane ( 5.0 mL), slowly dropwise added 1.6M n-butyllithium n-hexane solution (1.30 mL, 2.1 mmol) at -15°C, and continued to react at -15°C for 1 hour after the drop was completed.
[0032] Slowly add 3-phenylphosphineisocoumarin ethyl ester (286mg, 1.0mmol) in anhydrous 1,4-dioxane (5.0mL) dropwise to the above reaction solution, after the dropwise addition, warm to 0°C for reaction After 2 hours, water (10 mL) was added, extracted with ethyl acetate (3×10 mL), dried over anhydrous sodium sulfate, filtered, the solvent was evaporated under reduced pressure, and the residue was subjected to column chromatography (P:E=6:1-1: 1) 237 mg of white solid was obtained, which was the corresponding phosphine isocoumarinamide, and the yield was 70%. 1 H NMR: δ7.29-7.52(m, 9H), 6.52(s, 1H), ...
Embodiment 3
[0034]
[0035] Typical operation of sodium hydride (NaH) as a metal reagent: under nitrogen protection, add 60% NaH powder (264mg, 6.6mmol) and anhydrous tetrahydrofuran (THF, 5.0mL) into a 50mL dry round bottom flask, slowly Add nBuNH dropwise 2 (0.50mL, 5.0mmol), warmed up to room temperature to react for 1 hour after dropping.
[0036] Slowly add a solution of 3-phenyl-7-chlorophosphineisocoumarin ethyl ester (320mg, 1.0mmol) in anhydrous THF (5.0mL) dropwise to the above reaction solution at room temperature. After the dropwise addition, raise the temperature to 60°C for 3 hours , cooled to room temperature, added water (10mL), extracted with ethyl acetate (3×10mL), dried over anhydrous sodium sulfate, filtered, evaporated the solvent under reduced pressure, and the residue was subjected to column chromatography (P:E=6:1-1 : 1) 209 mg of white solid was obtained, which was the corresponding phosphine isocoumarin amide, and the yield was 60%. 1 H NMR: δ7.76-7.89(m, 3H),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com