2,2'-bialkoxyl-4,4'-biphenyl dicarboxylic acid and synthesis method thereof
A synthesis method and compound technology, applied in 2 fields, can solve the problems of low yield and the like, and achieve the effects of high yield, improved practicability, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
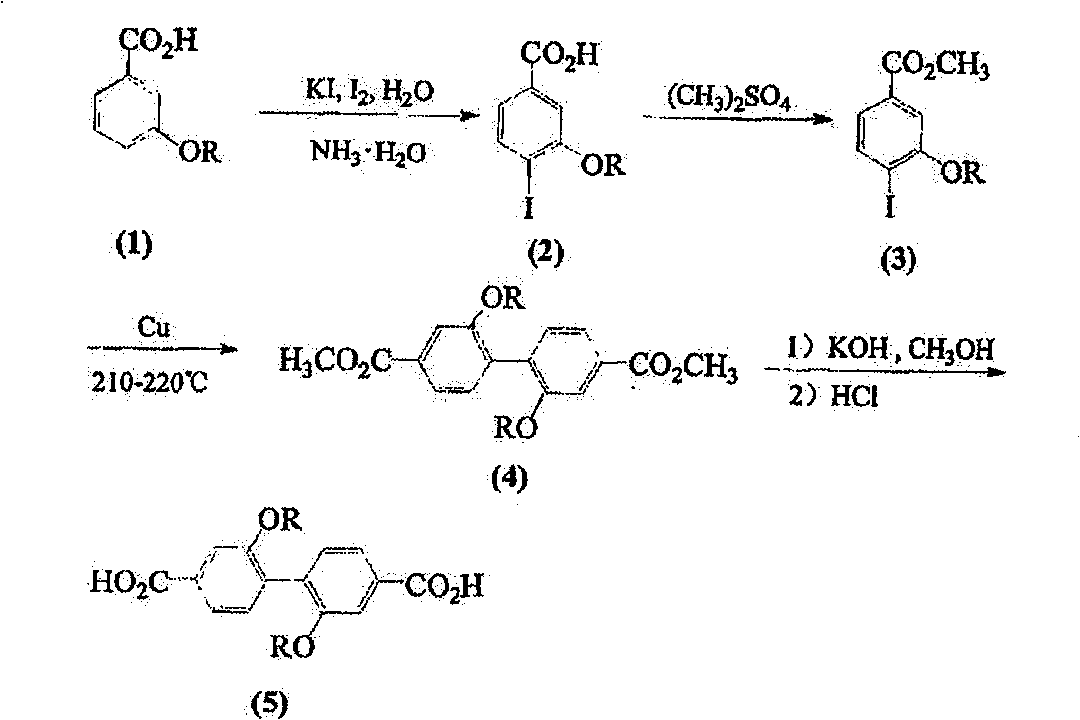
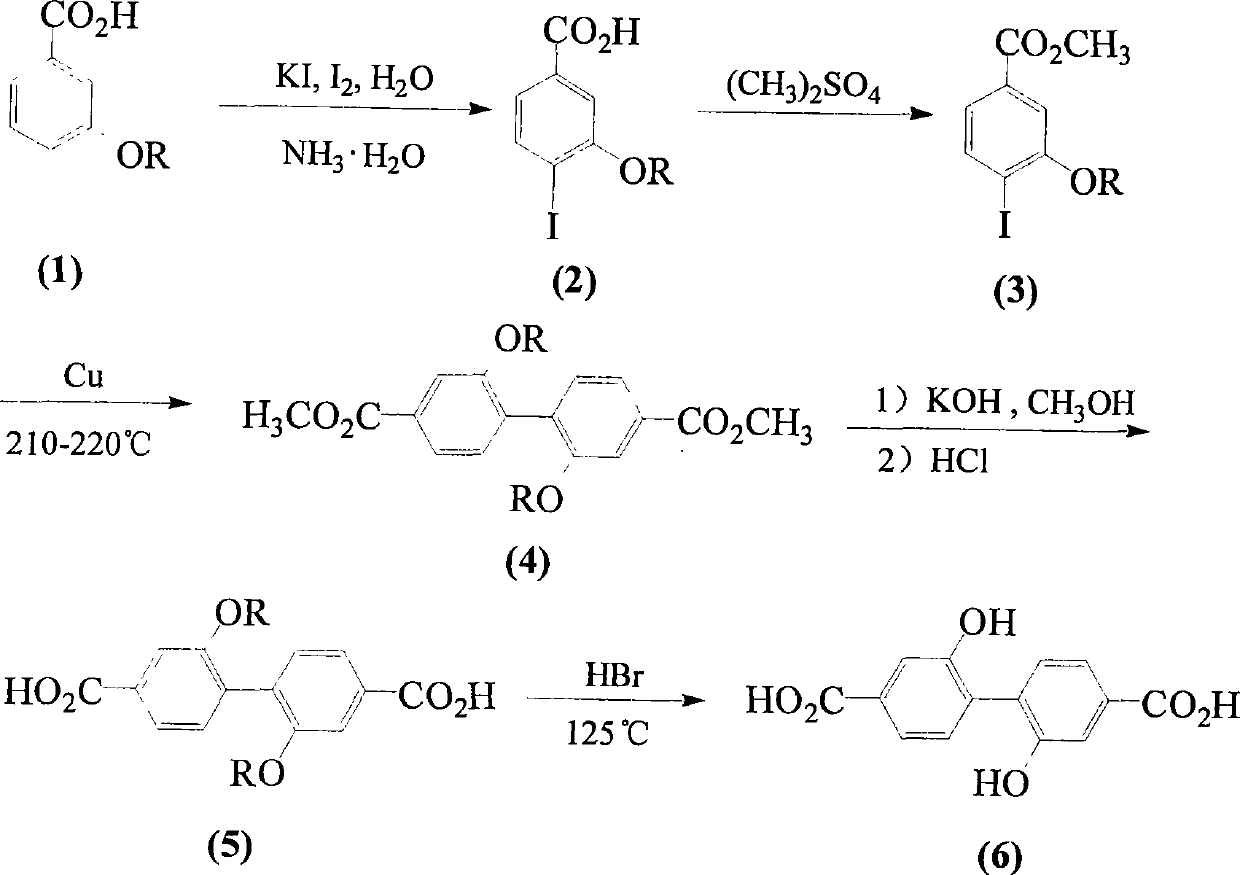
[0032] Example 1: Preparation of 2,2'-dimethoxy-4,4'-biphenyldicarboxylic acid
[0033] A, the preparation of 4-iodo-3-hydroxybenzoic acid
[0034] Iodine (7.82g, 30.7mmol) and KI (6.10g, 36.5mmol) were dissolved in 34mL of water, and it was added dropwise to the mass of 3-hydroxybenzoic acid (4.60g, 33.3mmol) within 15 minutes under stirring. The concentration is 26% ammonia water (67mL) solution, and continue to stir for 25 minutes. Acidify with 37% hydrochloric acid by mass percent, and separate the precipitate by suction filtration. The filtrate was extracted with ethyl acetate (60×3mL), and the solid separated from the extract was combined with the precipitate obtained by suction filtration, washed with water, and recrystallized to obtain white crystals of 4-iodo-3-hydroxybenzoic acid (4.30g, 16.4mmol). rate of 62%.
[0035] 1 H NMR (d 6 -DMSO): δ 7.13-7.14 (d, 1H), 7.42 (s, 1H), 7.79-7.81 (d, 1H).
[0036] B, the preparation of 4-iodo-3-methoxybenzoic acid methyl e...
Embodiment 2
[0045] Example 2: Preparation of 2,2'-dihydroxy-4,4'-biphenyldicarboxylic acid
[0046] Mix 2,2'-dimethoxy-4,4'-biphenyldicarboxylic acid (1.10g, 3.6mmol), hydrobromic acid (4mL) and glacial acetic acid (10mL), stir and reflux at 125°C for 8 hours , cooled, filtered with suction, washed with water, and dried to obtain 2,2'-dihydroxy-4,4'-biphenyldicarboxylic acid (C 14 h 10 o 6 ) (0.90 g, 3.3 mmol), the yield was 91.2%.
[0047] 1 H NMR (d 6 -DMSO): δ7.54-7.57 (d, 2H), 7.65 (s, 2H), 7.72-7.75 (d, 2H); elemental analysis (C 14 h 10 o 6 ): theoretical value (%), C, 61.32; H, 3.67; found value (%) C, 61.16; H, 3.86. m / z: 275.2 (M+1).
Embodiment 3
[0048] Example 3: Preparation of 2,2'-diethoxy-4,4'-biphenyldicarboxylic acid
[0049] A, the preparation of 4-iodo-3-ethoxybenzoic acid
[0050] Iodine (13.96g, 55mmol) and KI (10.46g, 63mmol) were dissolved in 70mL of water, and under stirring, it was added dropwise to 3-ethoxybenzoic acid (5.53g, 33.3mmol) at a mass percent concentration of 27 % ammonia (75mL) solution, the time was controlled at 20 minutes, and then continued to stir for 40 minutes. Acidify with 36% hydrochloric acid, and separate the precipitate by suction filtration. The filtrate was extracted with ethyl acetate (70×3mL), the solid separated from the extract was combined with the precipitate obtained by suction filtration, washed with water, and recrystallized to give white crystals of 4-iodo-3-ethoxybenzoic acid (4.57g, 15.7mmol) , yield 47.1%.
[0051] 1 H NMR (d 6 -DMSO): 1.29-1.33 (t, 3H), 3.90-3.97 (q, 2H), 7.35-7.39 (d, 1H), 7.47 (s, 1H), δ 7.73-7.75 (d, 1H).
[0052] The preparation of B, 4-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com