Medicine composition for treating chronic liver disease
A technology for chronic liver disease and composition, applied in the field of drugs for the treatment of chronic liver disease, can solve the problems of losing unique advantages, difficult to understand the ingredients contained, complex ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
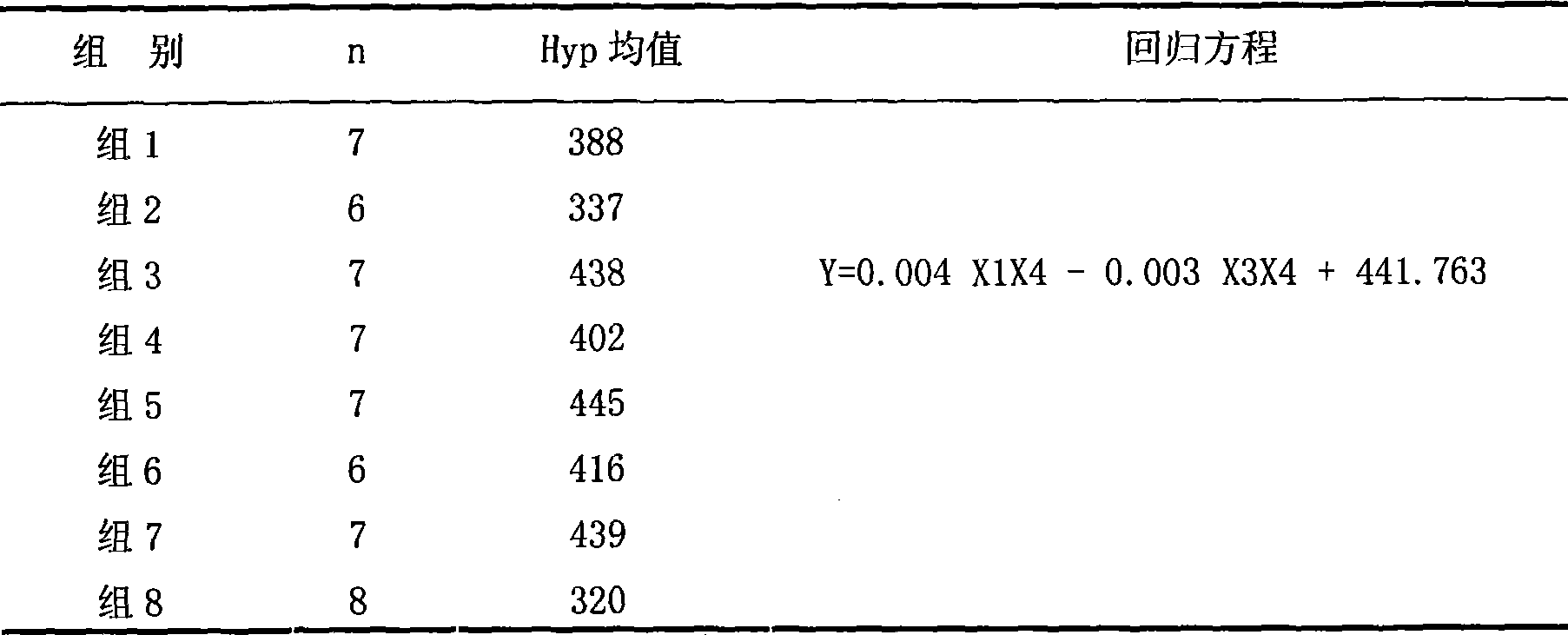
[0019] The corresponding optimal composition for reducing liver collagen content is obtained by using the DMN-induced rat liver fibrosis model and the uniform design method for regression analysis.
[0020] Materials and methods:
[0021] Animals: Wistar male rats, clean grade, body weight (160±10) g, provided by Shanghai Experimental Animal Center, Chinese Academy of Sciences.
[0022] Main reagents and drugs: Dimethylnitrosamine (DMN), product of Tokyo Chemical Industry Co., Ltd.; Cordyceps sinensis polysaccharide: derived from Cordyceps, product of Shanghai Kangzhou Fungal Polysaccharide Co., Ltd., specification 95%, batch number : 20060828; Salvianolic acid B: extracted from the traditional Chinese medicine Salvia miltiorrhiza, from the Shanghai Institute of Materia Medica, Chinese Academy of Sciences, with a content greater than 60%; Amygdaloside: derived from almonds, Xi'an Guanyu Biotechnology Co., Ltd. The company's product, specification 98%, batch number: 06061; Gyp...
Embodiment 2
[0033] Using carbon tetrachloride-induced rat liver fibrosis model and uniform design method regression analysis to obtain the corresponding optimal formula for reducing liver collagen content.
[0034] Materials and methods:
[0035] Animals: 90 Wistar male rats, SPF grade, weighing 170±15g, were provided by the Shanghai Experimental Animal Center of the Chinese Academy of Sciences.
[0036] Main reagents and drugs: carbon tetrachloride and olive oil were purchased from China Pharmaceutical Group. Cordyceps polysaccharide, salvianolic acid B salt, amygdalin, gypenosides are equivalent to "Example 1".
[0037] Uniform design drug screening experiment: 77 rats were randomly divided into normal group (7 rats) and model group (70 rats). Rats in the model group were injected subcutaneously with 100% CCl for the first time 4 Solution 3ml / kg, followed by 50% CCl 4Olive oil solution 2ml / kg, twice a week for 9 weeks. Rats in the normal group were injected with the same dose of ol...
Embodiment 3
[0048] Use the DMN-induced rat liver fibrosis model to verify the curative effect of the screened formulas A and B
[0049] Materials and methods:
[0050] Animals: SD male rats, clean grade, body weight (170±10) g, provided by Shanghai Experimental Animal Center, Chinese Academy of Sciences.
[0051] Main reagents and drugs: control drug Fuzheng Huayu Capsules (raw material, provided by Shanghai Modern Chinese Medicine Technology Development Co., Ltd.), liver function test kit (purchased from Nanjing Jiancheng Bioengineering Institute), other materials are the same as in "Example 1 ".
[0052] Grouping and modeling 60 SD rats were randomly divided into normal group (5) and modeling group (55). The modeling method was the same as in Example 1. After 4 weeks of modeling, 6 animals in the modeling group died. The remaining 49 model rats were randomly divided into model group (9 rats), A and B formula groups (8 rats each), A formula + salvianolic acid B group, B formula + salvi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com