Phthiobuzonum/diclothane compound topical formulation
A topical technology of phthalide, which is applied in the compound topical preparation of phthalide/dyclonine and its preparation to treat herpes simplex and herpes zoster skin diseases, so as to shorten the course of the disease, relieve the pressure of patients, The effect of improving the quality of life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1 Phthaloin / dyclonine ointment
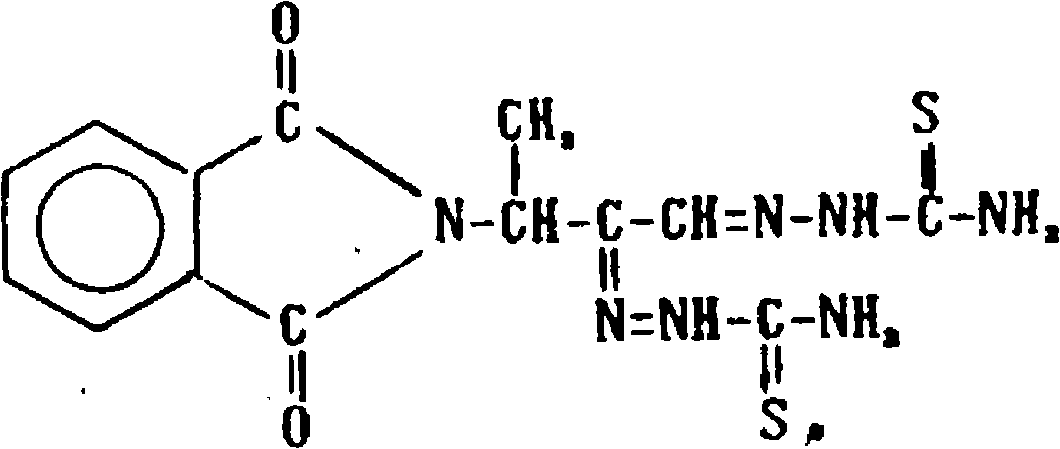
[0041] Phthalate 2g
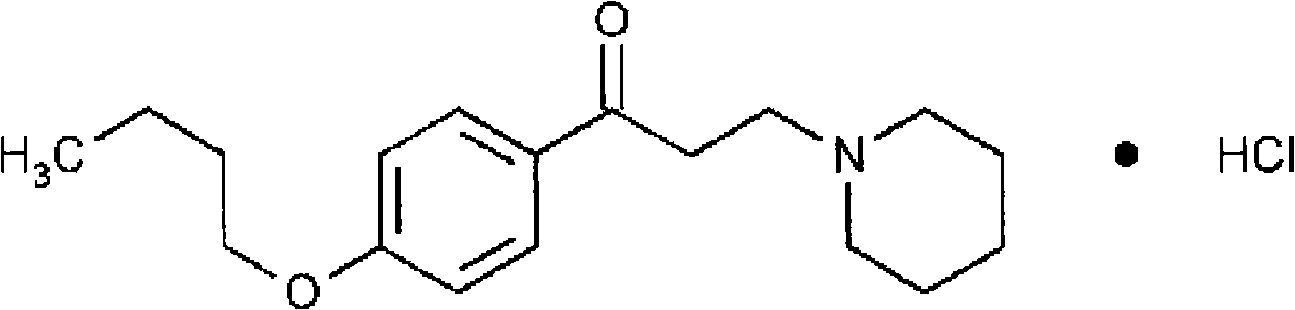
[0042] Dyclonine 1g
[0043] Dimethylformamide 5ml
[0044] Cetyl Alcohol 3g
[0045] Stearyl alcohol 3g,
[0046] Sodium Lauryl Sulfate 0.5g
[0047] Glycerol monostearate 7g
[0048] Distilled water 68ml
[0049] Glycerin 10ml
[0050] Preparation method: heat cetyl alcohol, stearyl alcohol, and glycerol monostearate, keep warm at 90 degrees, and heat sodium lauryl sulfate, glycerin, and water, and stir and mix with the above liquid to make a matrix, and then take The raw materials of phthalocyanine and dyclonine are dissolved in dimethylformamide, added to the prepared matrix, stirred and mixed well.
Embodiment 2
[0051] Example 2 Phthaloin / dyclonine gel
[0052] Phthalate 2g
[0053] Dyclonine 1g
[0054] Sodium Carboxymethyl Cellulose 3g
[0055] Propylene Glycol 10g
[0056] Dimethylformamide amount
[0057] Ethyl Nibelgin 0.1g
[0058] Add distilled water to 100g
[0059] Preparation method: Soak sodium carboxymethyl cellulose in 60ml of water for 24 hours for later use, and dissolve phthalocyanine and dyclonine with an appropriate amount of dimethylformamide, add them to sodium carboxymethyl cellulose soaked in water, Add propylene glycol, ethyl paraben and distilled water to the full amount and stir well.
Embodiment 3
[0060] Example 3 Phthaloin / dyclonine liniment
[0061] Phthalate 0.5g
[0062] Dyclonine 1g
[0063] Propylene Glycol 20ml
[0064] Azone 1ml
[0065] Triacetin add to 100ml
[0066] Preparation method: Mix the above-mentioned phthalodin, dyclonine, propylene glycol, azone, and an appropriate amount of triacetin, heat to dissolve, add triacetin to the full amount, and stir and mix well.
[0067] Similarly, the following examples are all expressed in terms of active ingredients and active ingredient ratios, and specific dosage forms and corresponding inactive ingredients refer to Example 1, or 2, or 3.
[0068]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com