Method for producing stable isotope of C-13 with chemical catalysis sorting by exchanging
A stable isotope and chemical catalysis technology, applied in the field of chemical catalytic exchange and separation of stable isotope 13C, can solve the problems of high energy consumption, high energy consumption and high cost, and achieve the effects of low energy consumption, high separation coefficient and small investment.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
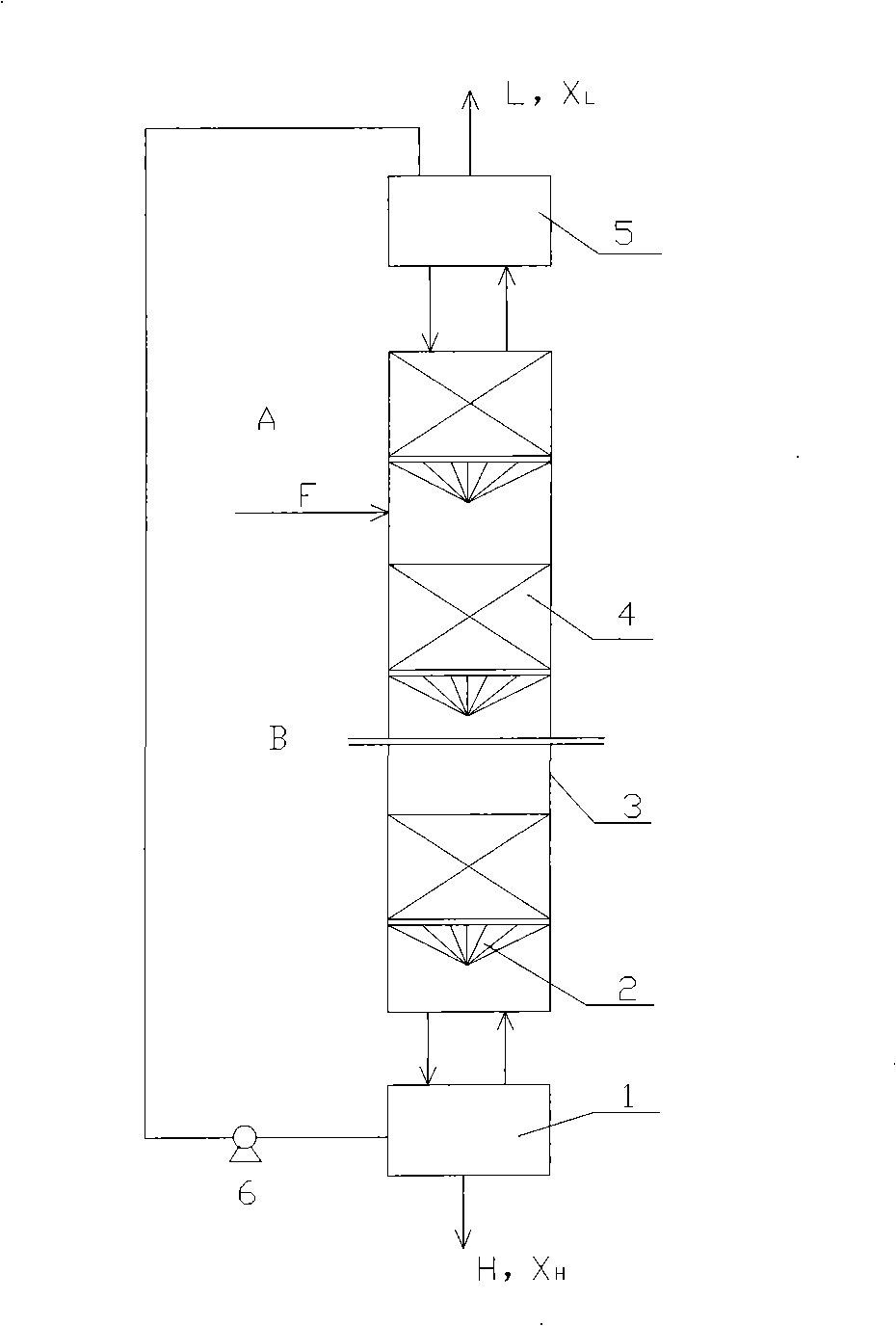
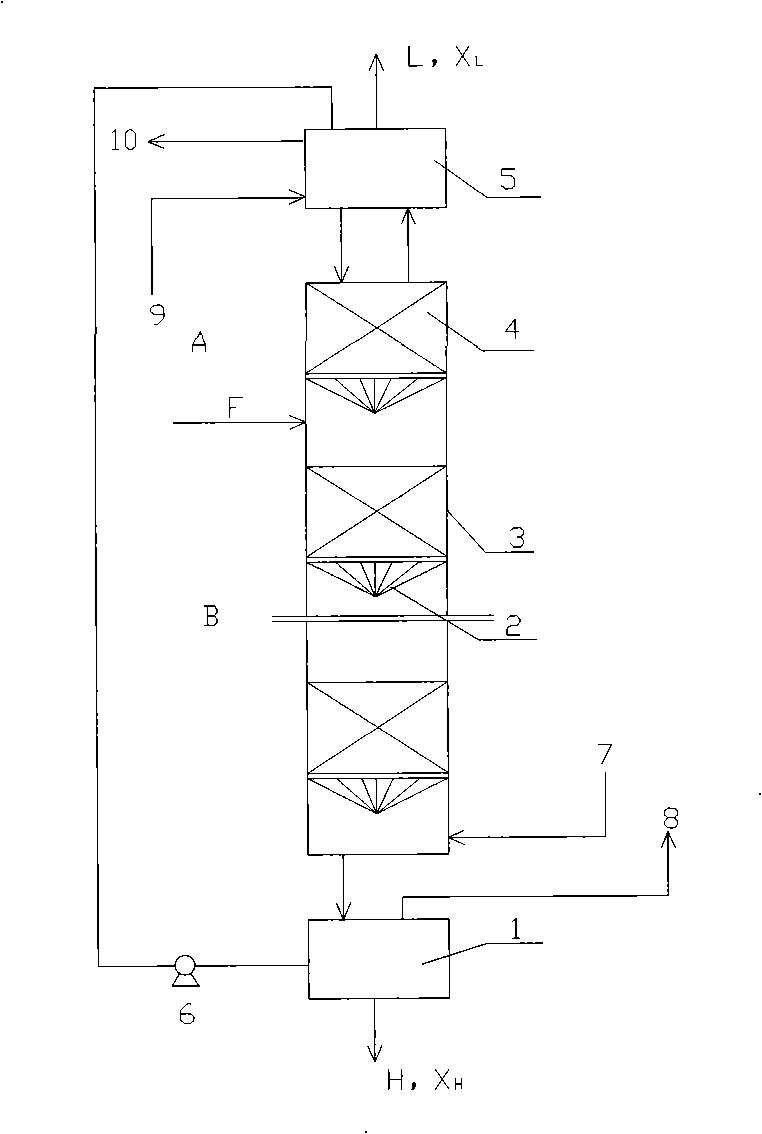
[0073] The isotope separation experiment was carried out in a single column with a height of 10m and an inner diameter of 5cm. The separation system is CO 2 / monoethanolamine / 80% methanol+20% triethylamine, the amine concentration is 1mol / l, and the solvent is a mixed solution of monoethanolamine and triethylamine. The packing is Helipak 3013 stainless steel. Raw material CO 2 It enters the exchange tower from F, and performs gas-liquid exchange with the descending carbamate solution. CO in the absorber 2 It is absorbed by the amine solution and converted into carbamate, which flows into the exchange tower, and the rising CO 2 For gas-liquid countercurrent exchange, heavy components 13 C is concentrated in the liquid phase and enriched in the bottom of the exchange column, the product 13 CO 2 Take it out of the decomposition tower. After the carbamate flows into the decomposition tower 1, it is decomposed by heat, and the released CO 2 Ascending into the exchange towe...
Embodiment 2
[0077] Using CO 2 / di-n-butylamine / octane chemical exchange system for separation of stable isotopes 13 c. The isotope separation experiment was carried out in a single column with a height of 10m and an inner diameter of 5cm. The separation system is CO 2 / di-n-butylamine / octane, the concentration of di-n-butylamine is 1.5mol / l, and the solvent is octane. The filler is a stainless steel wire wound around a rectangular helical coil. Raw material CO 2 It enters the exchange tower from F, and performs gas-liquid exchange with the descending carbamate solution. CO in the absorber 2 It is absorbed by the amine solution and converted into carbamate, which flows into the exchange tower, and the rising CO 2 For gas-liquid countercurrent exchange, heavy components 13 C is concentrated in the liquid phase and enriched in the bottom of the exchange column, the product 13 CO 2 Take it out of the decomposition tower. After the carbamate flows into the decomposition tower 1, it ...
Embodiment 3
[0081] Using CO 2 / di-n-butylamine / octane chemical exchange system for separation of stable isotopes 13 c. The isotope separation experiment was carried out in a single column with a height of 15m and an inner diameter of 0.6m. The separation system is CO 2 / di-n-butylamine / octane, the concentration of di-n-butylamine is 1.5mol / l, and the solvent is octane. Filled with 80 mesh CY700 stainless steel wire mesh corrugated packing, wire diameter 0.16mm, tooth profile angle 90°, peak height 3.5mm, specific surface area 750m 2 / m 3 . Raw material CO 2 It enters the exchange tower from F, and performs gas-liquid exchange with the descending carbamate solution. CO in the absorber 2 It is absorbed by the amine solution and converted into carbamate, which flows into the exchange tower, and the rising CO 2 For gas-liquid countercurrent exchange, heavy components 13 C is concentrated in the liquid phase and enriched in the bottom of the exchange column, the product 13 CO 2 Tak...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com