Tanshinone IIA polylactic acid nano particles and preparation method thereof
A technology of tanshinone and polylactic acid, which is used in pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc., can solve the problem that the treatment of patients is difficult to continue, the quality of life of cancer patients is decreased, and the mortality rate is increased. problems, to achieve the effect of increasing liver targeting, improving bioavailability, and changing in vivo processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Weigh 1.42mg of tanshinone IIA, 36.9mg of PLA, 80mg of lecithin and dissolve in a mixture of 4.5ml of dichloromethane and 0.5ml of acetone to form an organic phase, add to 30ml of F68 aqueous phase solution with a concentration of 1.0%, and add the organic phase to Put it into the water phase, sonicate the probe for 5 minutes in an ice bath to form an O / W emulsion, and then evaporate the organic solvent with magnetic stirring at room temperature to obtain a suspension of tanshinone IIA polylactic acid drug-loaded nanoparticles, which is filtered through a 0.65 μm microporous membrane to remove aggregation The body and the unwrapped drug are added with scaffold agent glucose and freeze-dried to obtain freeze-dried nanoparticles.
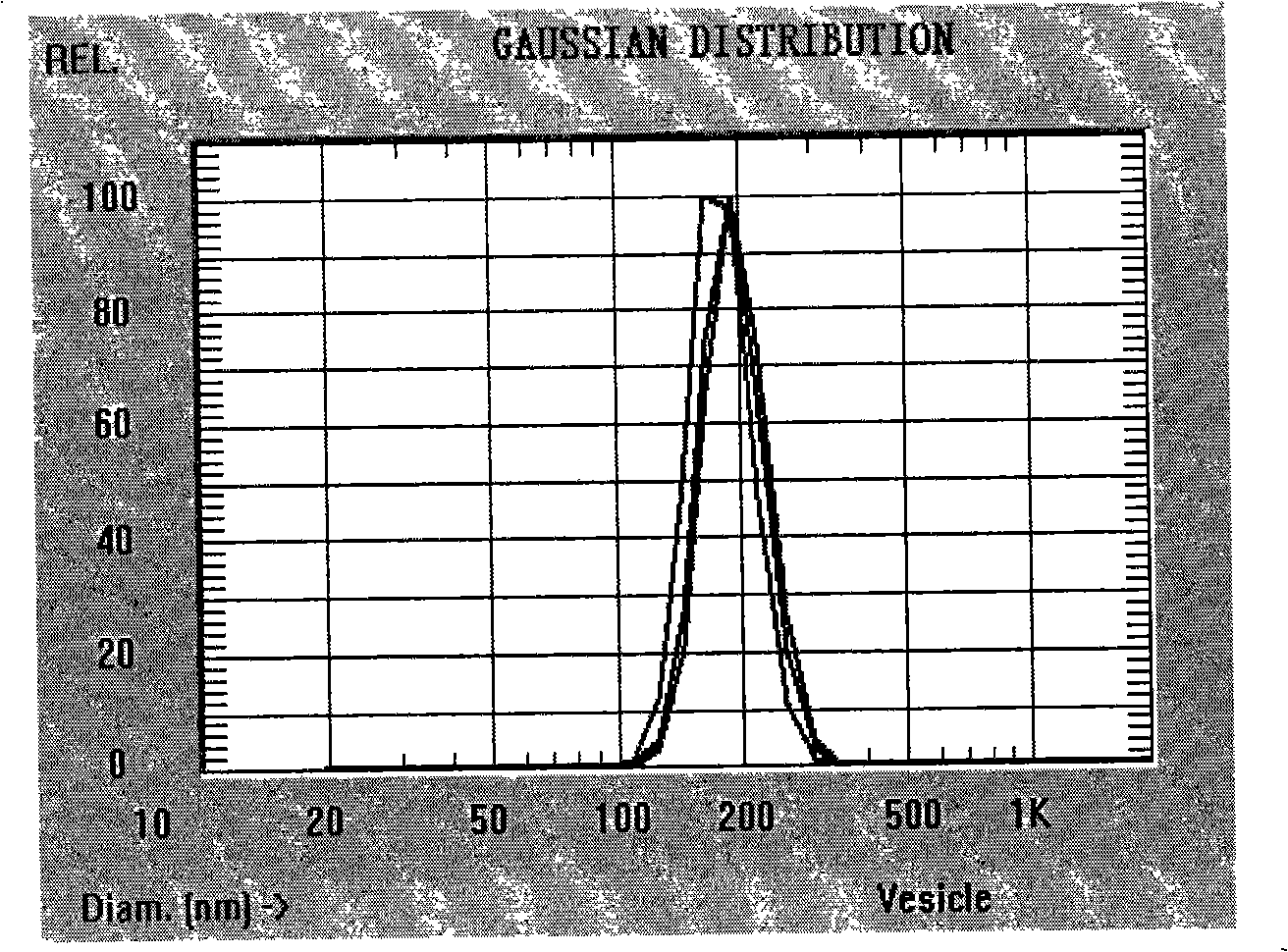
[0034] Detection: the average particle diameter of tanshinone II A polylactic acid drug-loaded nanoparticles is 210nm, and the encapsulation efficiency is 68%.
Embodiment 2
[0036] Take by weighing 2.58mg tanshinone IIA, 36.9mgPLA, 80mg lecithin and be dissolved in the mixed solution of 4.5ml dichloromethane and 0.5ml acetone, constitute the organic phase, add 30ml concentration and be the PVA aqueous phase solution of 2.0%, the organic phase is added to In the water phase, the probe was sonicated for 5 minutes in an ice bath to form an O / W emulsion, and then the organic solvent was evaporated by magnetic stirring at room temperature to obtain a suspension of tanshinone IIA polylactic acid drug-loaded nanoparticles, which was filtered through a 0.65 μm microporous membrane to remove aggregates And the uncoated medicine, adding scaffold agent lactose, freeze-drying to obtain freeze-dried nanoparticles.
[0037] Detection: the average particle diameter of tanshinone IIA polylactic acid drug-loaded nanoparticles is 230nm, and the encapsulation efficiency is 78%.
Embodiment 3
[0039] Weigh 1.5mg of tanshinone IIA, 100mg of PLA, 80mg of lecithin and dissolve in a mixture of 8ml of dichloromethane and 1ml of acetone to form an organic phase, add 30ml of F68 aqueous phase solution with a concentration of 1.5%, and add the organic phase to the aqueous phase , the probe was ultrasonicated for 5 minutes in an ice bath to form an O / W emulsion, and then the organic solvent was volatilized by magnetic stirring at room temperature to obtain a suspension of tanshinone II A polylactic acid drug-loaded nanoparticles, which was filtered through a 0.65 μm microporous membrane to remove aggregates and untreated particles. The encapsulated medicine is added with scaffolding agent mannitol, and freeze-dried to obtain freeze-dried nanoparticles.
[0040] Detection: the average particle diameter of tanshinone II A polylactic acid drug-loaded nanoparticles is 195nm, and the encapsulation efficiency is 82%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com