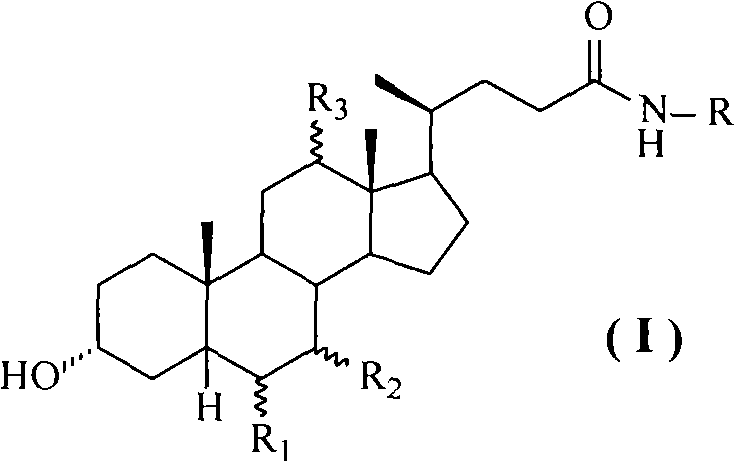
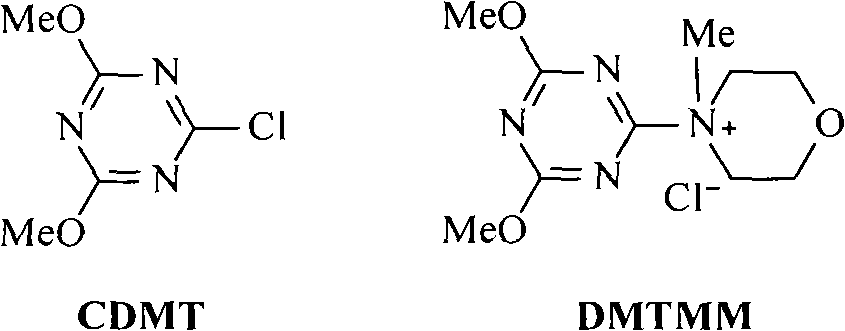
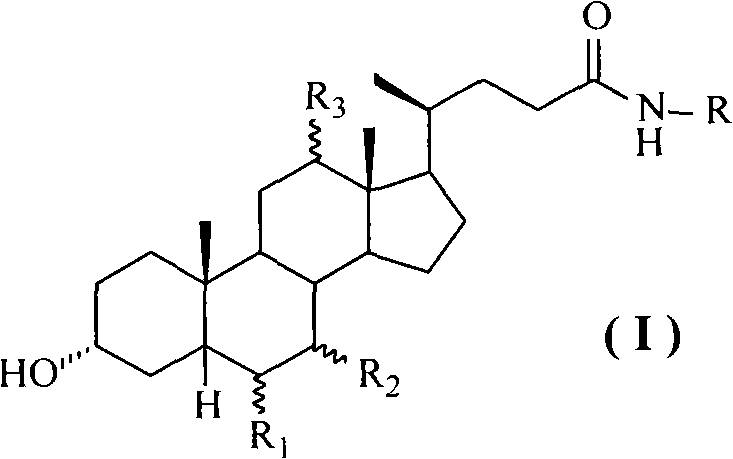
Method for preparing cholic acid conjugates
A technology of bile acid conjugates and conjugates, which is applied in the field of preparation of bile acid conjugates therapeutic drugs, can solve the problems of "three wastes" discharge, harsh reaction conditions, and low total yield, and achieve "three wastes" pollution less, mild reaction conditions, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The preparation of embodiment 1 taurodeoxycholic acid and its sodium salt
[0039] Add 39.26 g (0.1 mol) of deoxycholic acid, 13.77 g (0.11 mol) of taurine and 400 ml of isopropanol into the reaction flask, stir at room temperature until the solids are completely dissolved, add anhydrous K 2 CO 3 16.56 grams (0.12mol), after stirring evenly, add 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholine chloride in batches 35.98 gram (0.13mol), then stirred at room temperature and reacted for 12 hours. After the reaction, the insoluble matter was filtered off, and the filtrate was adjusted to pH 4-5 with 10% aqueous hydrochloric acid solution. Extract with 150 ml, separate the lower aqueous solution, cool in an ice bath, neutralize the aqueous solution with concentrated hydrochloric acid to strong acidity, continue stirring for 1 hour, filter the precipitated solid, and obtain 45.56 g of taurodeoxycholic acid white powder solid, mp183~ 185°C, (c=1.0, H 2 O), yield 88....
Embodiment 2
[0041] The preparation of embodiment 2 taurodeoxycholic acid and potassium salt thereof
[0042] The operation process is the same as in Example 1, except that isopropanol is replaced with deionized water, and chlorinated 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methyl Morpholine salt was replaced by 2-chloro-4,6-dimethoxy-1,3,5-triazine, anhydrous K 2 CO 3 Substitute 4-methylmorpholine to obtain 44.78 g of taurodeoxycholic acid white powder solid, mp182~186°C, (c=1.0, H 2 O), yield 86.5%. Take taurodeoxycholic acid and replace sodium hydroxide with potassium hydroxide to obtain potassium taurodeoxycholate with a yield of 92.5%.
Embodiment 3
[0043] Embodiment 3 Preparation of taurochenodeoxycholic acid and its sodium salt
[0044] The operation process is the same as in Example 1, except that deoxycholic acid is replaced by chenodeoxycholic acid, isopropanol is replaced by N,N-methylformamide, and anhydrous K 2 CO 3 Substituting triethylamine to obtain taurochenodeoxycholic acid as a white powder solid with a yield of 92.6%. The obtained taurochenodeoxycholic acid is salted with methanol solution of sodium methoxide to obtain sodium taurochenodeoxycholate, mp 180-182°C, yield 90.2%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mp | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com