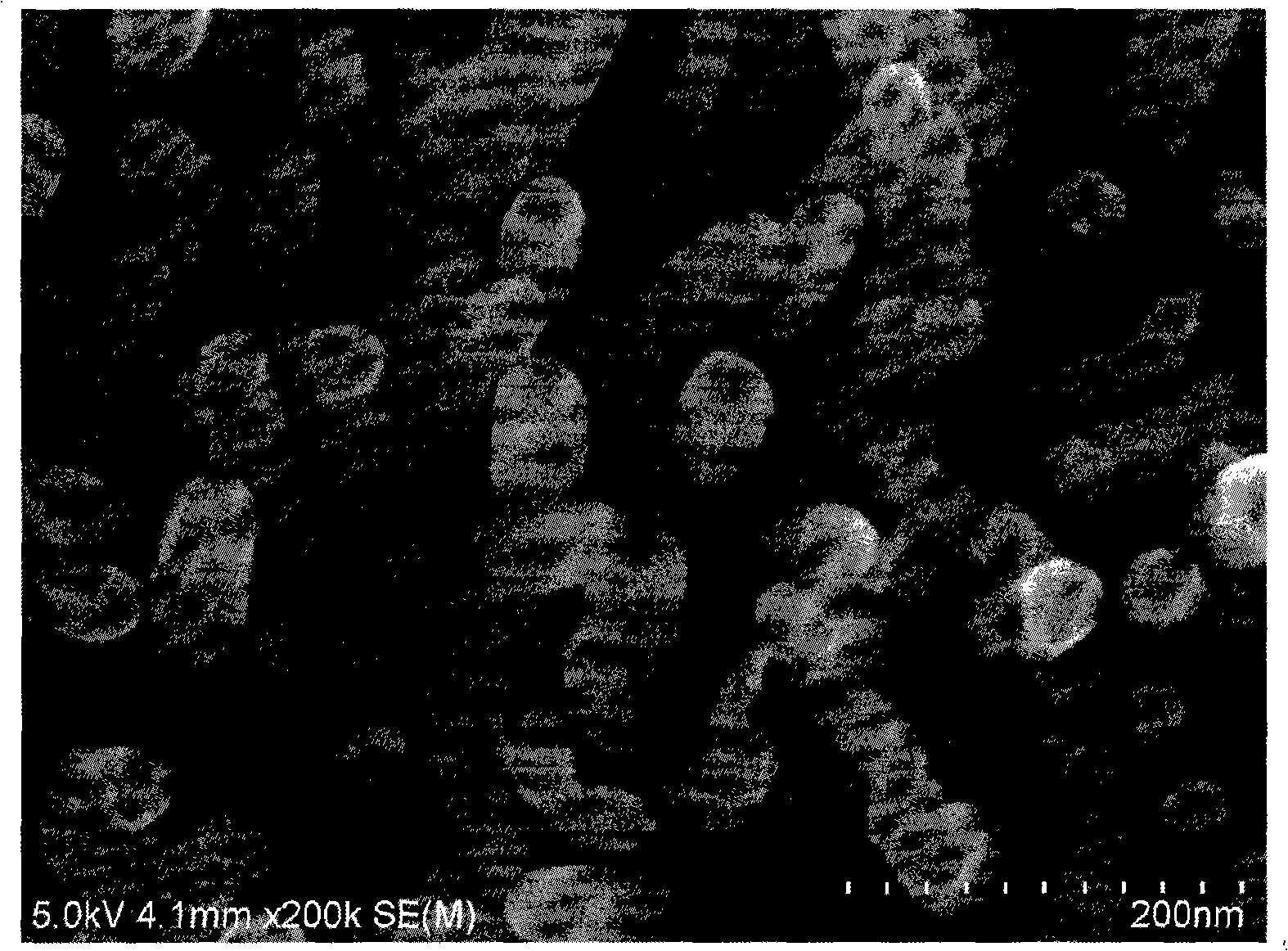
Chemical modification method of aromatic diazo salt to ZnO nanostructured surface
A chemical modification and nanostructure technology, applied in the field of surface chemistry, can solve the problems of large electron transport restrictions, achieve stable nanostructures, simple modification methods, and improve surface properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] (1) Cut the ZnO nanorod sample grown on the zinc substrate into a square sheet of 5mm*5mm, first soak the sheet in absolute ethanol to achieve complete immersion, ultrasonic treatment for 30 minutes, then take it out, and then use deionized Water was sonicated for 10 minutes, removed and dried under nitrogen for 10 minutes.
[0019] (2) the aromatic diazonium salt 2.93mg dissolved in 100mL acetonitrile dubbed 1*10 -4 mol / L solution, the ZnO nanorod flakes were soaked in the above solution for 24 hours under the condition of avoiding light.
[0020] (3) The above-mentioned ZnO nanorod flakes were taken out from the aromatic diazonium salt acetonitrile solution, and irradiated under a 250w ultraviolet lamp for 3 minutes.
[0021] (4) Wash in acetonitrile, acetone, and ethanol solvents for 2 minutes each to remove aromatic diazonium salts physically adsorbed on the surface of ZnO nanorods without participating in chemical reactions, and dry in a nitrogen environment for...
Embodiment 2
[0024] (1) Cut the ZnO nanorod sample grown on the zinc substrate into a square sheet of 5mm*5mm, first soak the sheet in absolute ethanol to achieve complete immersion, ultrasonic treatment for 30 minutes, then take it out, and then use deionized Water was sonicated for 10 minutes, removed and dried under nitrogen for 10 minutes.
[0025] (2) the aromatic diazonium salt 2.69mg dissolved in 100mL acetonitrile dubbed 1*10 -4 mol / L solution, the ZnO nanorod flakes were soaked in the above solution for 24 hours under the condition of avoiding light.
[0026] (3) The above-mentioned ZnO nanorod flakes were taken out from the aromatic diazonium salt acetonitrile solution, and irradiated under a 250w ultraviolet lamp for 3 minutes.
[0027] (4) Wash in acetonitrile, acetone, and ethanol solvents for 2 minutes each to remove the aromatic diazonium salt physically adsorbed on the surface of the ZnO nanorods without participating in the chemical reaction, and dry in a nitrogen envir...
Embodiment 3
[0029] (1) Cut the ZnO nanorod sample grown on the zinc substrate into a square sheet of 5mm*5mm, first soak the sheet in absolute ethanol to achieve complete immersion, ultrasonic treatment for 30 minutes, then take it out, and then use deionized Water was sonicated for 10 minutes, removed and dried under nitrogen for 10 minutes.
[0030] (2) the aromatic diazonium salt 3.79 mg was dissolved in 100 mL of acetonitrile to make a solution of 1*10-4 mol / L, and the ZnO nanorod flakes were soaked in the above solution for 24 hours in the dark.
[0031] (3) The above-mentioned ZnO nanorod flakes were taken out from the aromatic diazonium salt acetonitrile solution, and irradiated under a 250w ultraviolet lamp for 3 minutes.
[0032] (4) Wash in acetonitrile, acetone, and ethanol solvents for 2 minutes each to remove the aromatic diazonium salt physically adsorbed on the surface of the ZnO nanorods without participating in the chemical reaction, and dry in a nitrogen environment fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com