Novel biological substance nesfatin and its related substances and uses thereof
A technology of substances and related factors, applied in the direction of DNA/RNA fragments, cells modified by introducing foreign genetic materials, genetic material components, etc., can solve physiological and pharmacological effects without reporting, without showing NEF feeding regulation and/or body weight Reconciliation related reporting and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
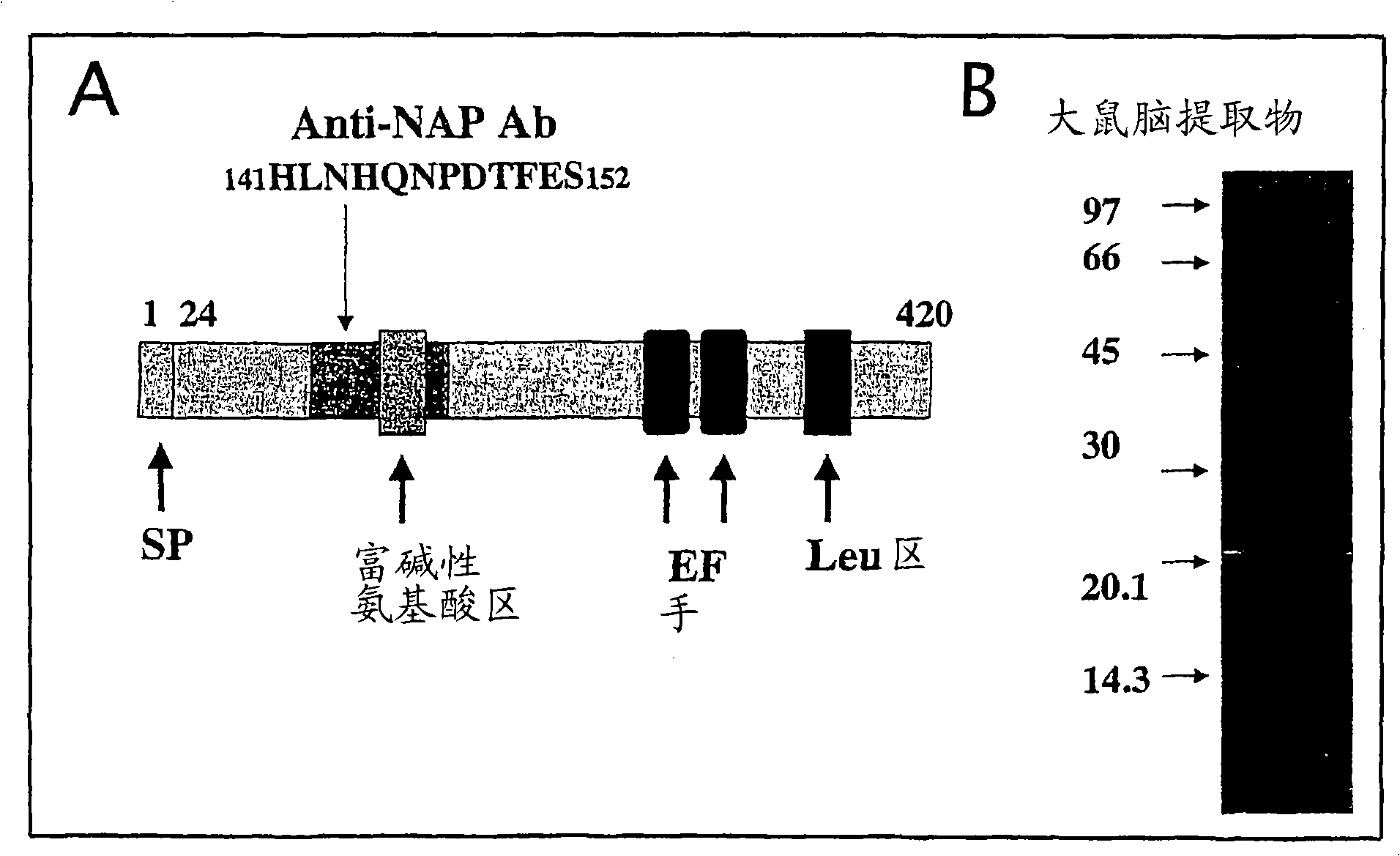
[0240] In the preparation method of the above-mentioned polypeptide of the present invention, in order to be suitable for expression, transcription, translation, etc. in transformants or transgenic non-human animals, the above-mentioned DNA sequence, plasmid, virus, etc. can be modified or changed more. For example, based on the synonymity of the genetic code (code), the entire protein coding region can be substituted with nucleotides. Such a sequence can be estimated from the amino acid sequence of a NESFATIN polypeptide or the like or the base sequence of a gene encoding the polypeptide, and assembled according to the following conventional synthesis methods. The above synthetic method can be carried out substantially according to the method of Itakura et al. (Itakura et al., Science 198, 1056, 1977) and the method of Crea et al. (Crea et al., Proc. Natl. Acad. Sci. USA75, 5765, 1978). Therefore, the gene encoding the above-mentioned NESFATIN polypeptide and the like used in...
Embodiment 1
[0320] Example 1 Cloning of genes induced by PPARγ agonist stimulation
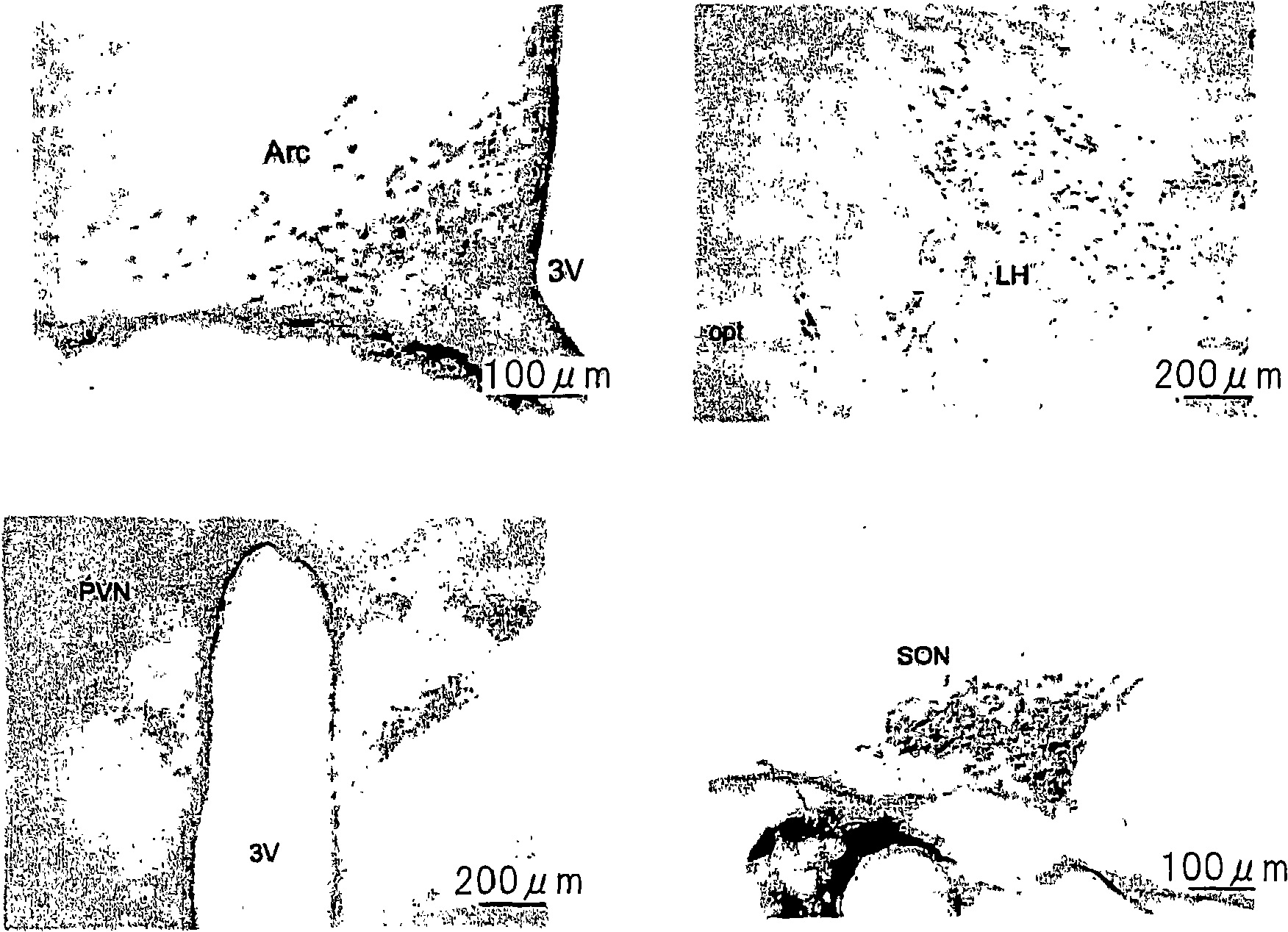
[0321] In order to identify genes whose expression is regulated by PPARγ and which act on feeding and / or body weight regulation, genes that are specifically induced when stimulated with PPARγ agonists in SQ-5 cells (mainly from human lung cancer cells) were cloned and extracted from Among them, genes having a possibility of functioning as secreted factors were screened.
[0322] The method for obtaining genes whose expression is specifically induced by PPARγ in non-small cell lung cancer can be carried out according to the method of Satoh et al., Oncogene (England), 2002, volume 21, pages 2171-2180. This method is briefly described below. SQ-5 (Riken Biological Resource Center (Riken Bioliso-Scentent) RBC0110) is in use for 10 -4 Troglitazone (Sankyo Co., Ltd.), one of the M PPARγ agonists, was stimulated to culture for 48 hours, and Poly(A)+RNA ( mRNA), double-stranded cDNA was prepared by reverse tra...
Embodiment 2
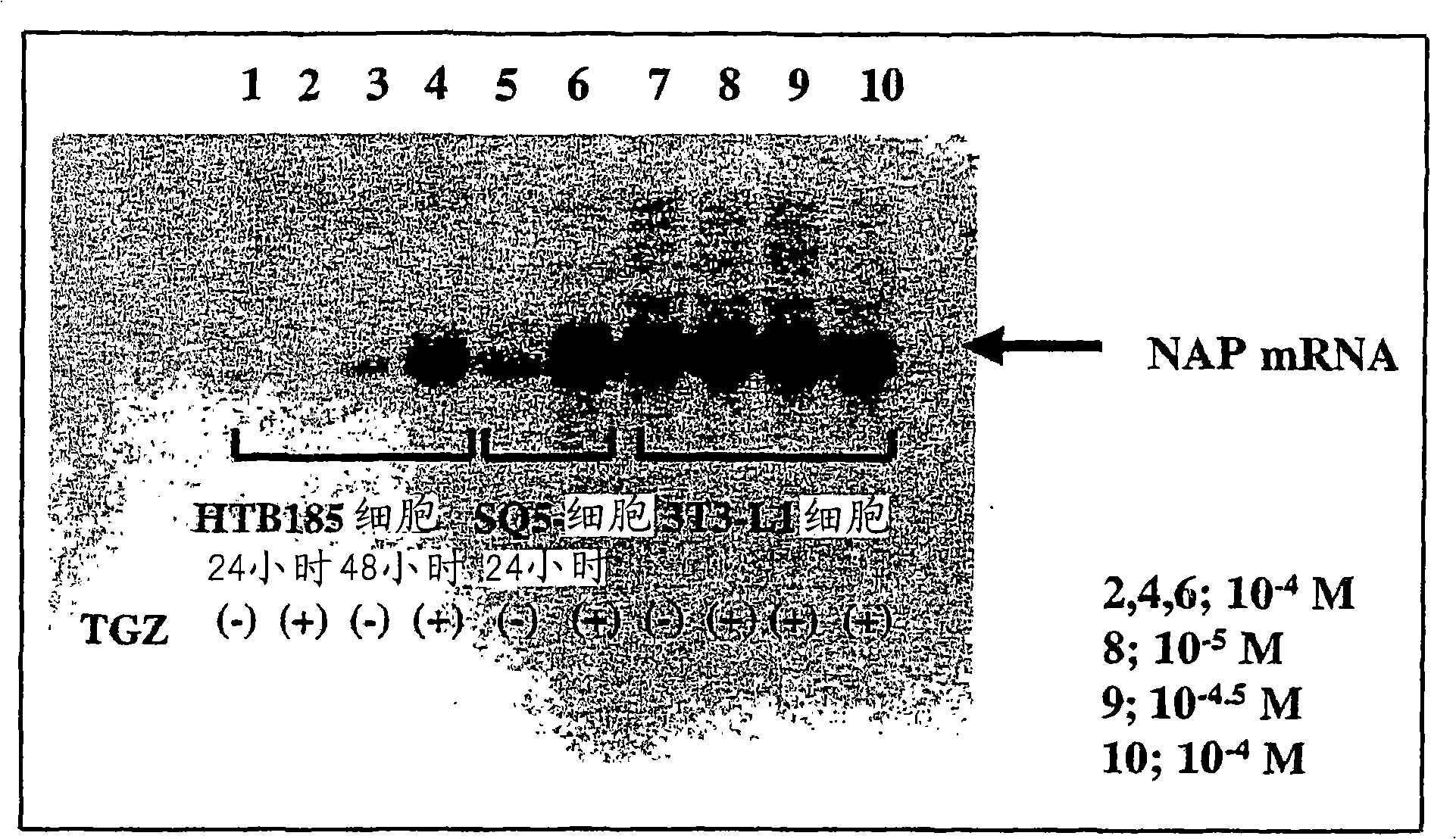
[0329] Example 2 Stimulation with PPARγ agonist to induce expression of NEFA gene in cultured cell lines
[0330] In order to confirm the induction of the NEFA gene by troglitazone, the expression analysis of the NEFA gene was performed by Northern blotting using the PPARγ-expressing cell lines HTB185 and SQ5 and the adipocyte line 3T3-L1.
[0331] SQ-5 (Riken Bioresource Center RBC0110) was obtained by subculture with RPMI1640 medium (Inbitologien-BIL, product number 11875-085) containing 10% fetal bovine serum (Inbitologien-GIBGO). Human brain medulloblastoma cell line HTB185 (D283Med: ATCC HTB185) and mouse embryonic fibroblast cell line (preadipocyte cell line) 3T3-L1 cell line (ATCC CL-173) induced by insulin, dexamethasone, and IBMX The DMEM medium containing 10% fetal bovine serum (Invitrogen-GIBCO, product number 11995-040) was used for subculture. 10 suspended in 10ml medium 6 Each cell was seeded in a 10 cm culture dish, the cells were allowed to attach to the matr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com