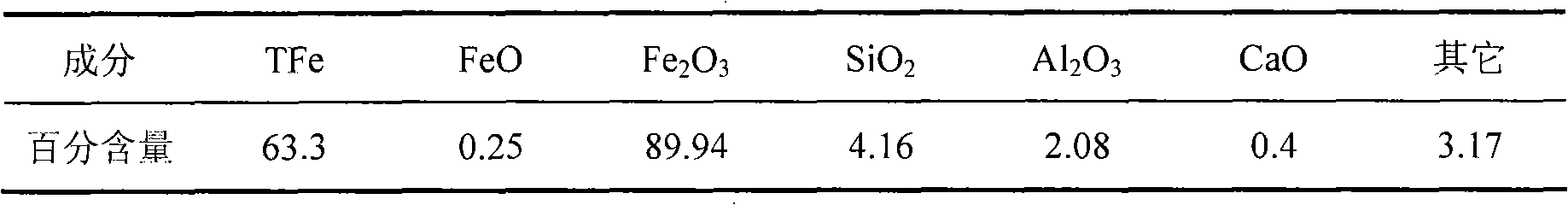Method for preparing iron sulfate with iron ore
A technology of iron ore and ferric sulfate, applied in the direction of ferric sulfate, etc., can solve the problems of high consumption of oxidant and catalyst, high equipment investment, high maintenance cost, limited production and application of ferric sulfate, etc., and achieves easy control of production process and operating conditions. , good economic benefits, significant economic and social benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Embodiment 1: measure 100ml mass percent concentration and be that the sulfuric acid solution of 40% is in the 500ml four-necked flask that has agitator; After iron ore is ground into-200 purpose iron ore powder, put into the muffler furnace in 800 Calcined at ℃ for 2h, after cooling, weigh 8.17g of iron ore powder, add it into the above-mentioned four-neck flask with sulfuric acid, react in a constant temperature water bath at 100℃ for 2h, cool and filter, use sulfosalicylic acid as indicator , EDTA complexometric titration method measured [Fe 3+ ]concentration. Ferric sulfate solution can be obtained, where [Fe 3+ ]=0.85mol / L.
Embodiment 2
[0014] Embodiment 2: measure 100ml mass percentage concentration and be that the sulfuric acid solution of 40% is in 500ml four-necked flasks with stirring device; After iron ore is ground into-200 purpose iron ore powder, put into the muffler furnace in 900 Calcined at ℃ for 2h, after cooling, weigh 8.17g of iron ore powder, add it into the above-mentioned four-neck flask with sulfuric acid, react at 100℃ for 2h, cool and filter with sulfosalicylic acid as indicator, EDTA [Fe] was determined by complexometric titration 3+ ]concentration. Ferric sulfate solution can be obtained, where [Fe 3+ ]=0.86mol / L.
Embodiment 3
[0015] Embodiment 3: measure 100ml mass percentage concentration and be that the sulfuric acid solution of 40% is in the 500ml four-necked flask that has agitator; After iron ore is ground into-200 purpose iron ore powder, put into the muffler furnace at 800 Calcined at ℃ for 3h, after cooling, weighed 8.17g of iron ore powder, added to the above-mentioned four-necked flask with sulfuric acid, reacted at 100℃ for 2h, cooled and filtered with sulfosalicylic acid as indicator, EDTA [Fe] was determined by complexometric titration 3+ ]concentration. Ferric sulfate solution can be obtained, where [Fe 3+ ]=0.86mol / L.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com