LMP-1 recombinant gland related viral vectors as well as construction method and uses thereof
A viral vector, LMP-1 technology, applied in the direction of virus/phage, virus, application, etc., can solve the problems of low virus titer, unstable recombinant virus, limited capacity of therapeutic genes, etc., and achieve the effect of broad application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1. Construction and detection of recombinant adeno-associated virus vector rAAV / LMP-1 and rAAV / mLMP-1
[0048] Materials and their sources:
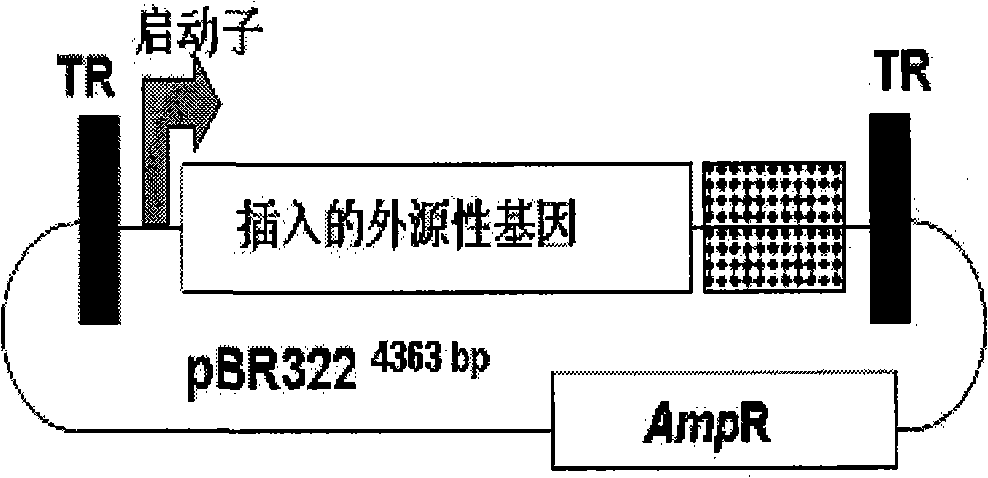
[0049] A. The pBR322 plasmid (named pBR-AAV2) carrying the whole genome DNA of AAV type 2: prepared by Professor Paul L. Hermonat, one of the main technical directors of the United States Broadway Gene International Co., Ltd. (Hermonat, P.L., and Muzyczka, N. Use of adeno-associated virus as a mammalian DNA cloning vector: transduction of neomycin resistance into mammalian tissue culture cells. Proc. Natl. Acad. Sci. U.S.A. 81: 6466-6470.).
[0050] B. Human nasopharyngeal carcinoma cells: isolated from cancer tissues of nasopharyngeal carcinoma patients or purchased from commercial sources, immunohistochemistry confirmed LMP-1 positive.
[0051] C. The pCI-neo plasmid carrying the CMV promoter was purchased from Promega Corporation in the United States, and the plasmid pSG424 carrying the SV40 early promoter was purchase...
Embodiment 2
[0062] Embodiment 2, preparation of recombinant adeno-associated virus (rAAV) and virus titer determination
[0063] Materials and their sources:
[0064] A. The recombinant adeno-associated virus vector rAAV / LMP-1 carrying the LMP-1 gene constructed in Example 1 and the recombinant adeno-associated virus vector rAAV / mLMP-1 carrying the LMP-1 mutant gene (rAAV / AmLMP-1, rAAV / BmLMP-1, rAAV / CmLMP-1 and rAAV / DmLMP-1).
[0065] B. Auxiliary plasmid pHelper containing the Rep gene and Lip / Cap gene of AAV: constructed by Professor Liu Yong from the Gene Therapy Center of the University of Arkansas Medical College Hospital (Liu, Y., Chiriva-Internati, M., Grizzi, F.Salati , E., Roman, J.J., Lim S., and Hermonat, P.L. Rapid induction of cytotoxic T cell response against cervical cancer cells by humanpapillomavirus type 16 E6 antigen gene delivery into human dendritic cells by an adeno-associated virus vector.py Cancer 8: Gene Thera 948-957.).
[0066] C. AAV-HEK293 cells containing ...
Embodiment 3
[0086] Example 3. Tumor killing experiment of introducing tumor-associated antigen into monocyte-macrophage-dendritic cell line
[0087] Materials and their sources:
[0088] A. rAAV virus: rAAV / LMP-1 and rAAV / mLMP-1 (rAAV / AmLMP-1, rAAV / BmLMP-1, rAAV / CmLMP-1).
[0089] B. AIM-V cell culture medium: purchased from Invitrogen, USA.
[0090] C. Cytokines: colony cell stimulating factor (GM-CSF), interleukins 2, 4, 7 (IL-2, 4, 7) and tumor necrosis factor (TNF-α) were purchased from American R&D Corporation.
[0091] 1. Tumor killing experiments
[0092] like Figure 5 As shown, the whole process of the tumor-killing experiment based on one or more rAAV viruses carrying tumor-associated antigen genes (LMP-1 gene and mutant gene thereof) of the present invention infecting tumor patient's monocytes includes the following steps:
[0093] A. Take 50-150 ml of peripheral blood from a tumor patient, use a blood cell separator (or lymphocyte separation medium) to obtain peripheral bl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com