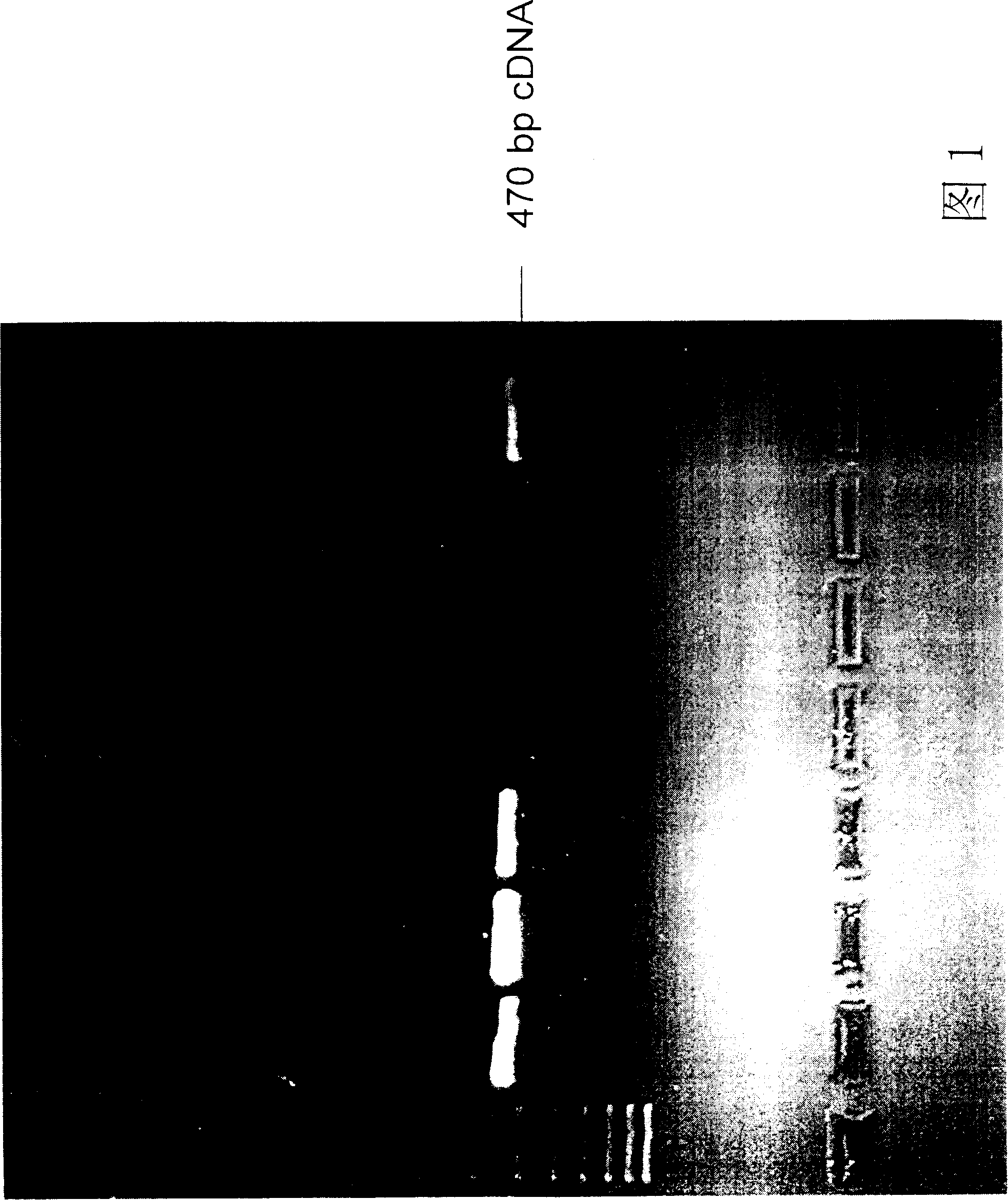
Preparation and application of humanized anti-spasmotoxin monoclone antibody
A monoclonal antibody, tetanus toxin technology, applied in the application, antibody, botanical equipment and methods, etc., to avoid pollution, long-acting drug concentration effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] Example 1: Obtaining and screening human-derived plasma cells that secrete anti-tetanus toxin antibodies.
[0100] 100 ml of peripheral blood was obtained from a healthy 20-year-old male donor who had recently been immunized with tetanus vaccine. The blood donor had no history of any chronic disease, had no disease symptoms at the time of blood donation, had been immunized against tetanus 15 days before, and had not received any other vaccines in the past year. The whole blood was treated with EDTA and centrifuged through a Ficoll density gradient at 3000 rpm for half an hour. Blood leukocytes were obtained by aspirating the middle layer of Ficoll. Put the white blood cell suspension into the cell culture bottle and incubate at 37 degrees for 1 hour to remove the mononuclear cells adhering to the plastic surface. Anti-CD19 antibody-conjugated magnetic beads (Dynabeads) were mixed with washed non-adherent leukocytes (20 magnetic beads / 1×10 8cells / ml) and incubated for...
Embodiment 2
[0101] Example 2: Cloning of variable region gene fragments of human anti-tetanus toxin antibody.
[0102] First, the cells of the plasma cell clones screened in Example 1 were dissolved with a solution containing NP-40 and an RNase inhibitor at 4° C. for 10-30 minutes. Cell lysates are mixed with buffer containing reverse transcriptase. The mRNA of each clone was individually converted into a cDNA library by a reverse transcription biochemical reaction. The details are as follows: the reverse transcription reaction conditions are 65° C. for 3 minutes, slowly lower the temperature to 22° C., and keep warm for 3 minutes. Add fresh buffer containing reverse transcriptase. Incubate at 38°C for 100 minutes. Afterwards, it was incubated at 65°C to terminate the reverse transcription reaction.
[0103] Design a set of primers as shown below to clone the light and heavy chains of the antibody. VH primers include:
[0104] GGG AAT TCC CAT GGA CTG GAC CTG GA
[0105] GGG AAT TCA...
Embodiment 3
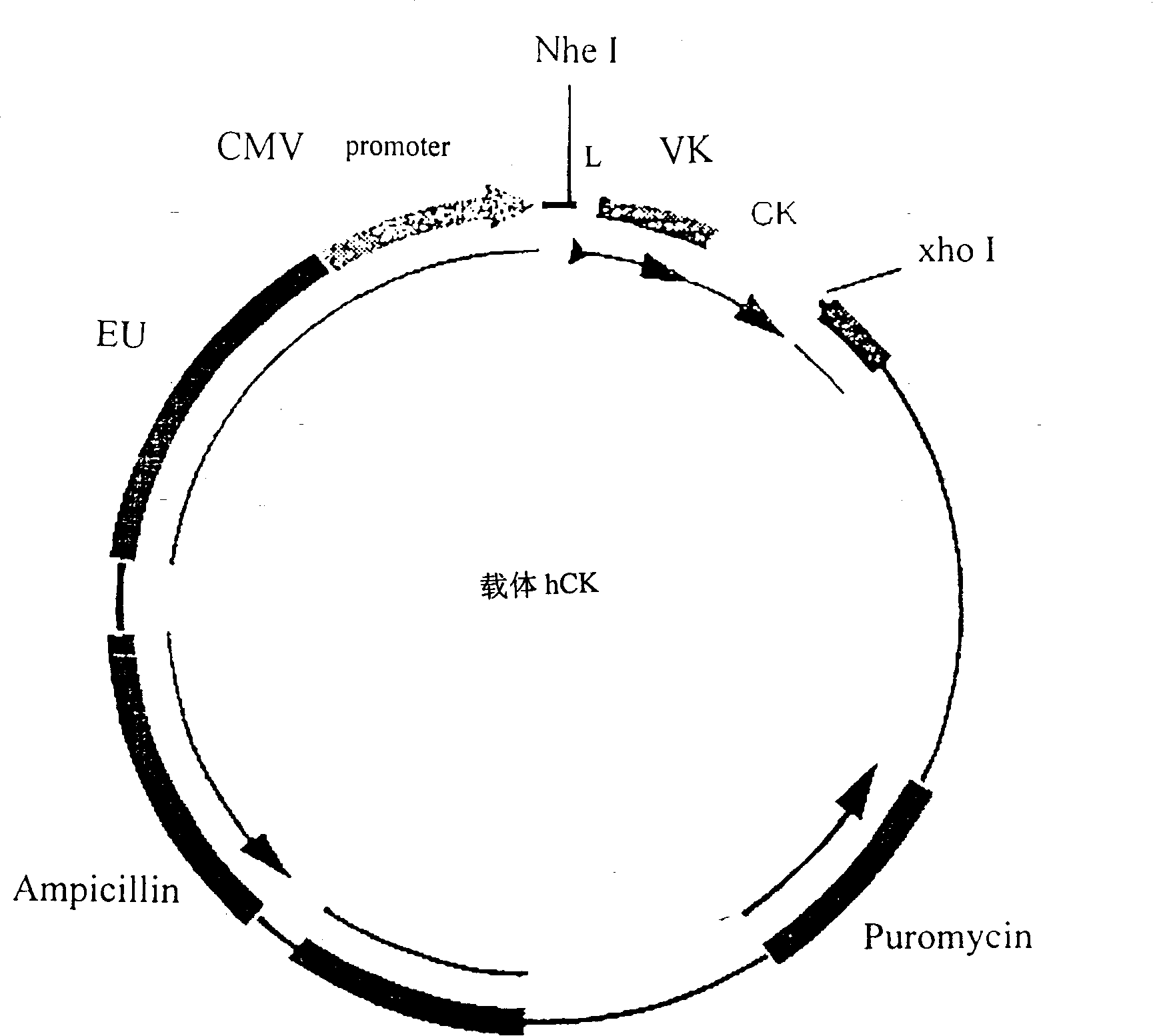
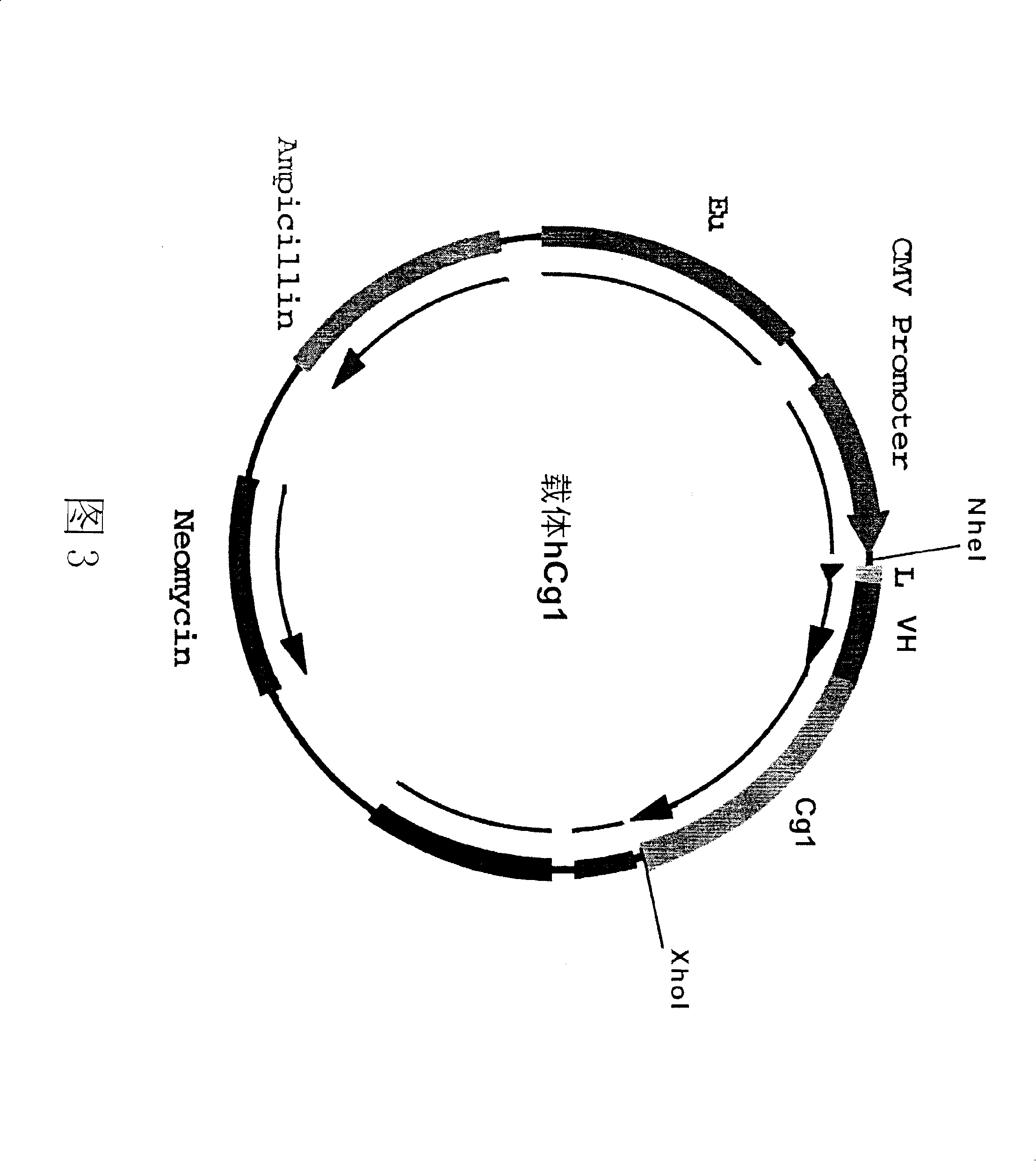
[0118] Example 3: Construction of the expression vector of human anti-tetanus toxin antibody.
[0119] figure 2 and Figure 3 is a schematic diagram of the antibody heavy chain carrier hCg1 and the antibody light chain carrier hCK. In these two vectors, we choose CMV as the promoter and EU as the enhancer. The size of the vector is about 7000bp. In both vectors, the constant region IgG of the antibody IgG heavy chain γ The cDNAs of the constant region k of 1 and the light chain have been respectively connected to the downstream of the promoter in the vector CMV. The heavy chain and light chain variable region gene fragments of the antibody cloned in Example 1 and attached to the pCR2.1 plasmid vector were excised with specific restriction enzymes, and separated and purified. The variable region genes of the heavy chain and light chain were respectively connected to two different expression vectors: the variable region gene of the light chain was connected to the antibody li...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com