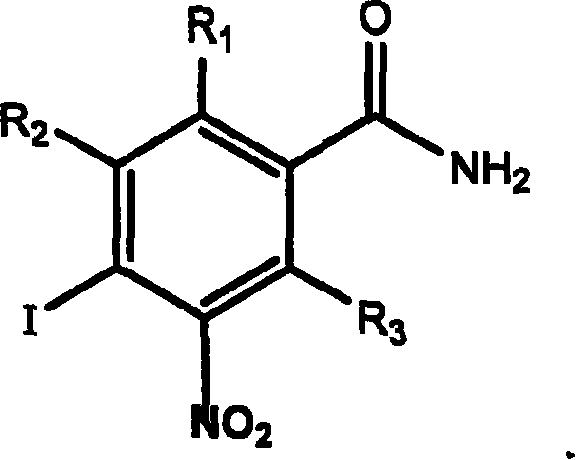
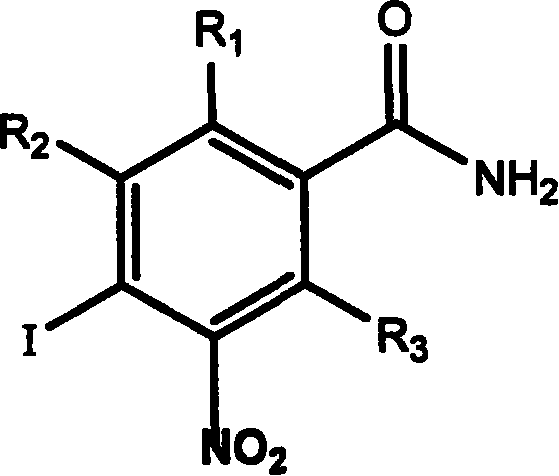
Application of aromatic nitro compound in preparing medicine for treating virus hepatitis
A technology of viral hepatitis and aromatic nitro, which is applied in the direction of antiviral agents, drug combinations, digestive system, etc., can solve the problems of unreported application and achieve the effect of inhibiting proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Synthesis of nitroaromatic compounds
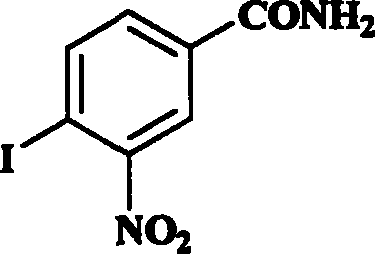
[0030] Preparation of 4-iodo-3-nitrobenzamide
[0031]1025mg of 4-iodo-3-nitrobenzoic acid (provided by Chemica Alta Ltd., Edmonton, Alberta, Canada) was dissolved in 10ml of N,N-dimethylformamide, cooled to 10°C, then 0.76ml of chlorinated Sulfoxide, stirred at room temperature for 1 hour, then added to 20ml of pre-cooled concentrated ammonia water to obtain a black-yellow mixture, stirred for 5 minutes, added 50ml of pre-cooled deionized water, a bright yellow precipitate appeared, and cooled in an ice bath for 10 minutes , the precipitate was filtered off with suction, rinsed with cold water and then dried in vacuo to obtain 500.4 mg of crude product, which was recrystallized from acetonitrile to obtain 415.2 mg of 4-iodo-3-nitrobenzamide, melting point: 152-155°C. HNMR (DMSO) δ 7.67 (bs, 1H, a non-equivalent proton in the amide group), 7.84, 7.85 and 7.86, 7.87 (dd, 1H, the 5th hydrogen of the aromatic ring), 8.22 an...
Embodiment 2
[0032] Example 2: In vitro experimental study of anti-hepatitis B
[0033] Material
[0034] 4-iodo-3-nitrobenzamide was prepared with DMSO to make an appropriate concentration in the experiment, and the culture medium was diluted 3 times during the test, with a total of 8 dilutions.
[0035] Positive control drug: Lamivudine was produced by Glaxo Wellcome.
[0036] method
[0037] Drug-to-cytotoxicity test HepG2.2.15 cells were dispersed into a single cell suspension with 0.6% trypsin, and the concentration was 3 × 10 in DMEM medium containing 10% fetal bovine serum. 5 Cell suspension of cells / ml, seeded at 0.1ml / well in a 96-well plate, placed at 37°C, saturated humidity, 5% CO 2 Culture in an incubator, and change 0.1 ml / well of drug-containing culture medium after 2 days, with four wells for each concentration. The drug stock solution was serially diluted to 8 concentrations. Set to 37℃, saturated humidity, 5%CO 2 The culture was continued in the incubator, and a con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com