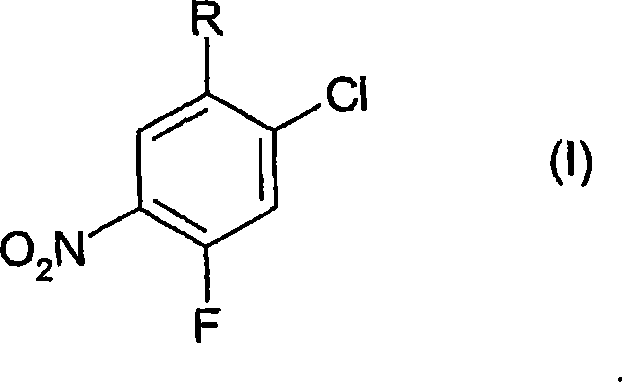
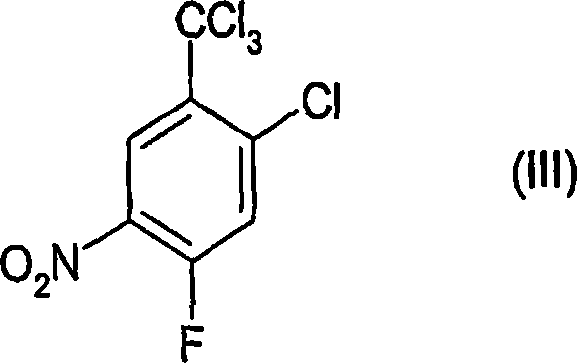
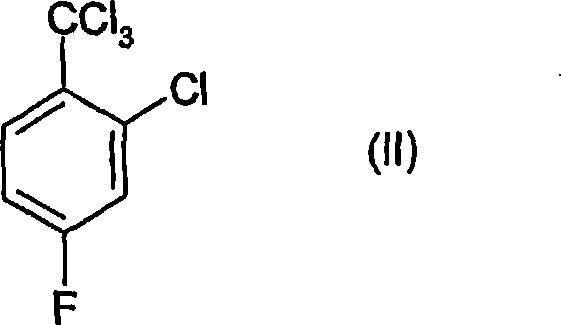Process for the preparation of benzoic acid derivatives via a new intermediate of synthesis
A technology of derivatives, nitrobenzoic acid, applied in the field of novel synthetic intermediates, can solve problems such as intractability, a large amount of sulfuric acid, and multiple impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Preparation of 2-chloro-4-fluoro-5-nitrobenzotrichloride (formula (III)) (according to step (a))
[0060] To a 500ml 4-neck round bottom flask equipped with a mechanical stirring device, cooling device, thermometer and dropping funnel was added 150.2g of 30% oleum and 60.2g of concentrated nitric acid (99%) while maintaining the temperature below 10°C . To the sulfuric acid-nitric acid mixture thus obtained was added 115.6 g of 2-chloro-4-fluorotrichlorotoluene over about 2.5 hours, while maintaining the temperature between 0-10°C. The reaction was allowed to stand at this temperature for an additional 2 hours to complete, then poured into an ice / water mixture and a white solid was immediately observed to precipitate. To this mixture was added about 300 g of dichloromethane and the phases were separated, the organic phase was washed with NaHCO 3 The solution was washed, then dried over magnesium sulfate, filtered and evaporated to dryness. 132 g of the title produc...
Embodiment 2
[0079] Preparation of 2-chloro-4-fluoro-5-nitrobenzotrichloride (formula (III)) (according to step (a))
[0080] A 500 ml 4-neck cryogenically cooled round bottom flask equipped with a mechanical stirring device, thermometer, dropping funnel and acid gas trap was charged with 602.8 g of a mixture of sulfuric acid and nitric acid containing 22% nitric acid, and the temperature was cryogenically cooled to 0°C. 251.8 g of 2-chloro-4-fluorotrichlorotoluene were added through the dropping funnel while maintaining the temperature between 0-2°C. The addition was completed within 4.5 hours and with good stirring, the resulting mixture had a pale yellow color. The reaction mixture was placed at 0-4°C for an additional 2 hours to allow the reaction to complete. The reaction was monitored by GC, and as the reactants disappeared, the acid was then diluted with 150.4 g of deionized water. The process was carried out using external cooling in order to keep the temperature below 10°C. ...
Embodiment 3
[0084] Preparation of 2-chloro-4-fluoro-5-nitrobenzoic acid (according to step (b))
[0085] 41.6g 96% H in a 250ml reactor 2 SO 4 , and 8.4g of water was added dropwise (to adjust the acid concentration to 80%). The temperature of the acid was adjusted to about 70°C and the portionwise addition of 10 g of purified 2-chloro-4-fluoro-5-nitrotrichlorotoluene (titer by GC = 99.5%) was started. The temperature was then adjusted to 100-110°C, noting the gradual formation of a white solid product dispersed in the mixture. This temperature was maintained for about 3 hours, then the reaction mixture was cooled to about 50°C and diluted with 100 g of water, and the product was then extracted with 40 g of ethyl acetate at room temperature. The phases were separated and the organic solution was washed with 40 g of water. Finally, evaporation of the solvent gave 7 g of the title product as a white solid.
[0086] Titer by HPLC = 94.5%, molar yield 88%.
[0087] Melting point (°C):...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com