All-human source follicle stimulating hormone beta single-chain antibody screening method and its uses
A follicle-stimulating hormone and single-chain antibody technology, applied in biochemical equipment and methods, material inspection products, measuring devices, etc., can solve the problems of unfavorable large-scale production, short half-life, and high cost of mouse-derived monoclonal antibody preparation, Achieve convenient amplification and production, reduce immunogenicity, and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1 Screening of fully human anti-FSH-β single-chain antibody
[0053] 1. Preparation of Antigen, Antibody Library and Related Bacterial Strains
[0054] 1) Synthesize the FSH-β fragment, synthesized by the Institute of Biochemistry and Cell Biology, Shanghai Chinese Academy of Sciences, with a full length of 21 amino acid residues (33-53 amino acid residues of the FSH-β subunit). Amino acid residue sequence:
[0055] Glu-Glu-Cys-Arg-Phe-Cys-Ile-Ser-Ile-Asn-Thr-Thr-Trp-Cys-Ala-Gly-Tyr-Cys-Try-Thr-Arg, (valley-valley-cysteine- Ethyl-phenylpropanoid-cysteine-isoluminous-silk-isoluminous-asparagus-threo-threo-color-cysteine-propyl-glycerin-cysteine-tyrosine-threo-arthrin), molecular weight 2.5Kda, synthetic purity 99.8%. The synthesized FSH-β fragment is in the form of white crystalline powder, soluble in water.
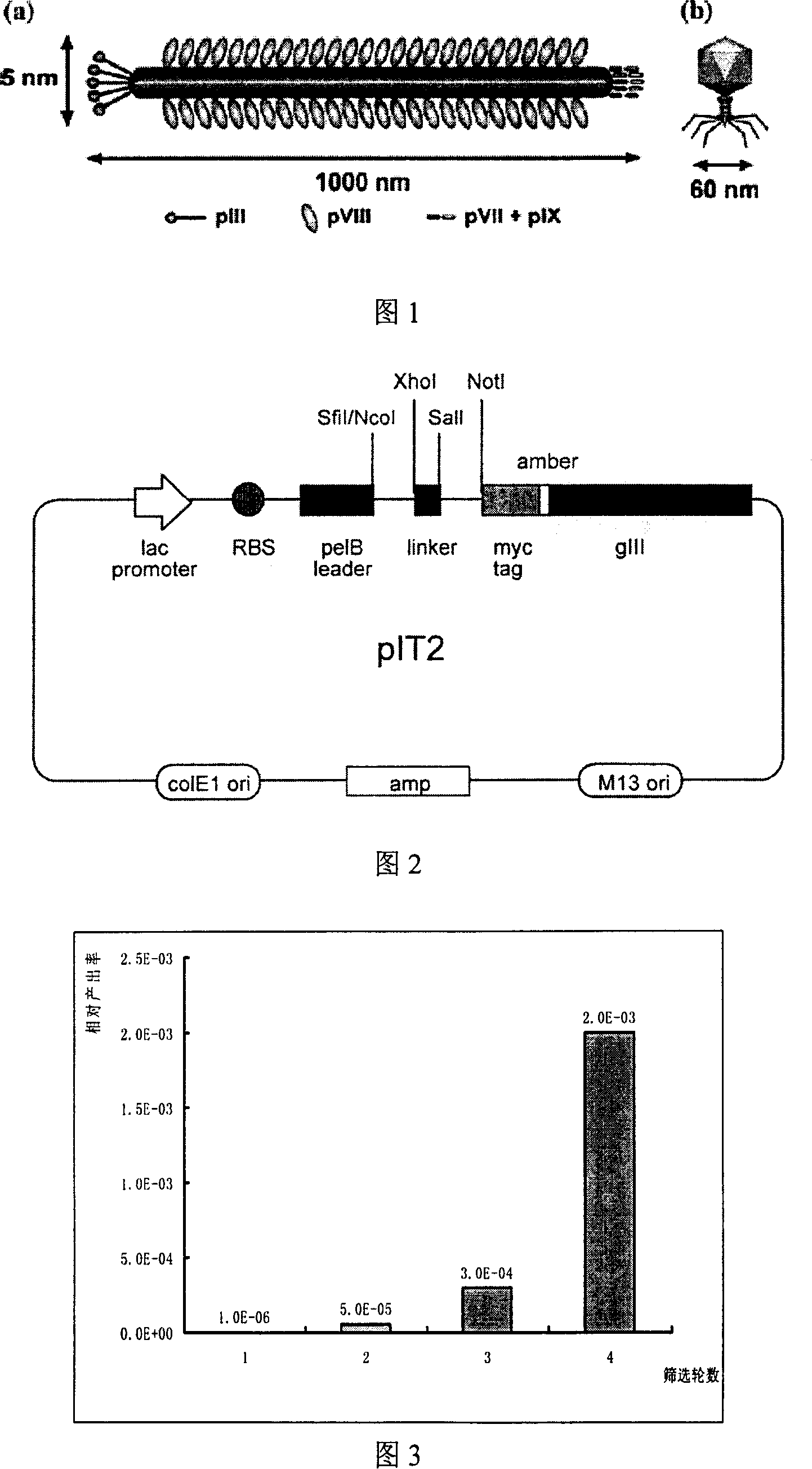
[0056] 2) Fully human single-chain phage antibody library: the library capacity is about 1×10 8 , pIT2 phagemid vector, including gene III, myc tag an...
Embodiment 2
[0125] Example 2 Identification of immune activity of FSH-β phage antibody
[0126] 1. ELISA identification of anti-FSH-β phage antibody
[0127] Method I
[0128] 1) The bacteria obtained in the last round of screening were diluted and spread on TYE culture plates (with a final concentration of 100 μg / ml Amp), and cultured overnight at 37°C.
[0129] 2) Add 100 μl of 2×TY medium to each well of the 96-well plate (containing a final concentration of 100 μg / ml Amp+1% glucose), and randomly pick 90 colonies from the TYE culture plate to inoculate the 96-well plate (the remaining 6 wells No bacteria), 250rpm, 37°C shaking culture overnight.
[0130] 3) Take another 96-well plate, add 200 μl of 2×TY medium (containing a final concentration of 100 μg / ml Amp+1% glucose) to each well, and transfer 5 μl of bacterial solution from each well of the first 96-well plate to the second Plates were shaken at 250 rpm at 37°C for 2 hours. Add glycerol to each well of the first 96-well plate ...
Embodiment 3
[0322] Embodiment 3 Anti-FSH-β single chain antibody identification
[0323] method
[0324] 1) Menopausal women urine 4000ml overnight, refer to Table 8 to extract total protein in urine by salting out with saturated ammonium sulfate.
[0325] Table 8 Saturated ammonium sulfate salting-out protein table
[0326] Ammonium sulfate final concentration at 25°C, % saturation
ammonium initial
strong
Spend,
% full
Harmony
10
20
25
30
33
35
40
45
50
55
60
65
70
75
80
90
100
The number of grams of solid ammonium sulfate added per 1000ml solution
0
56
114
144
176
196
209
243
277
313
351
390
430
472
516
561
662
767
10
57
86
118
137
150
183
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com