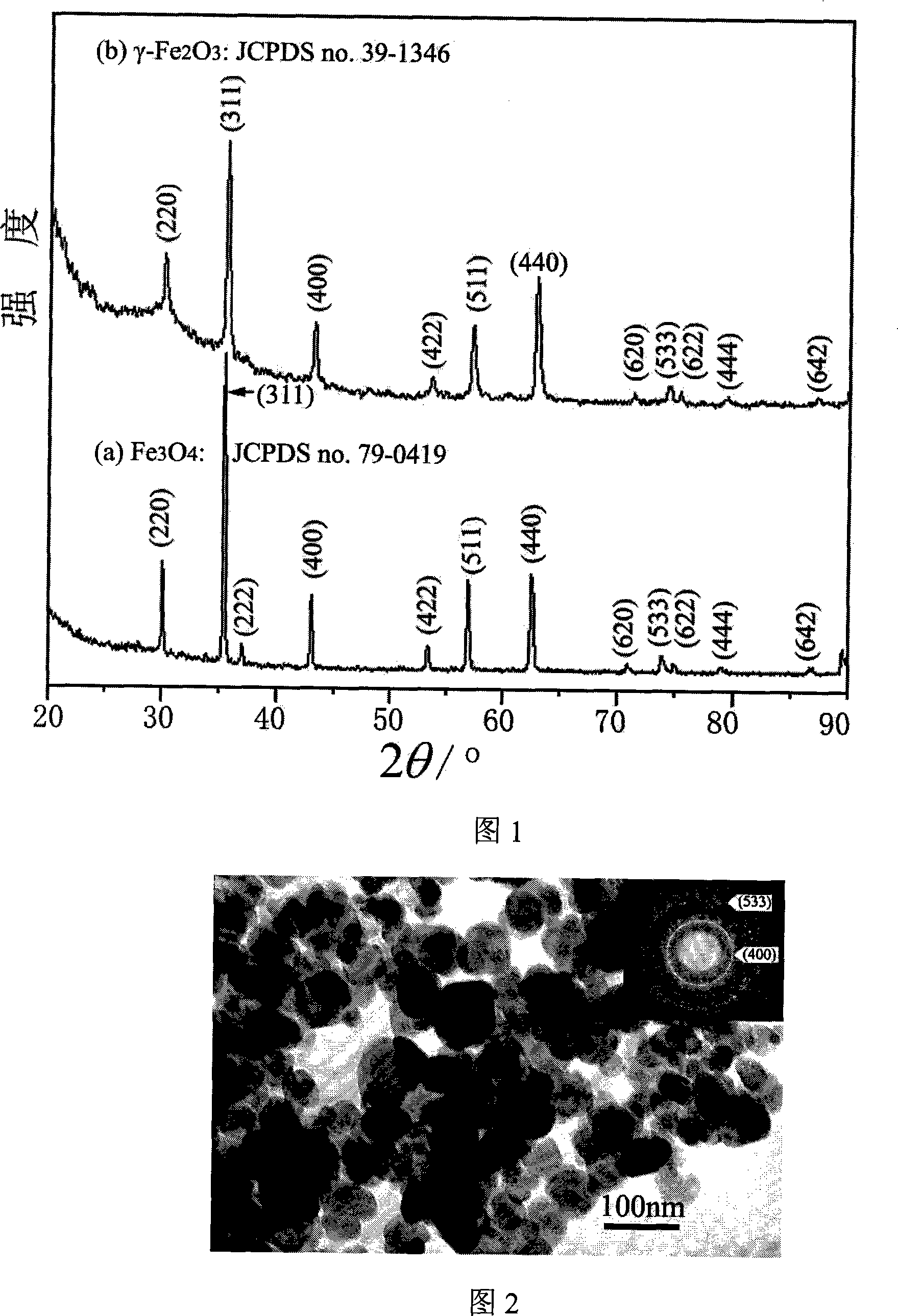
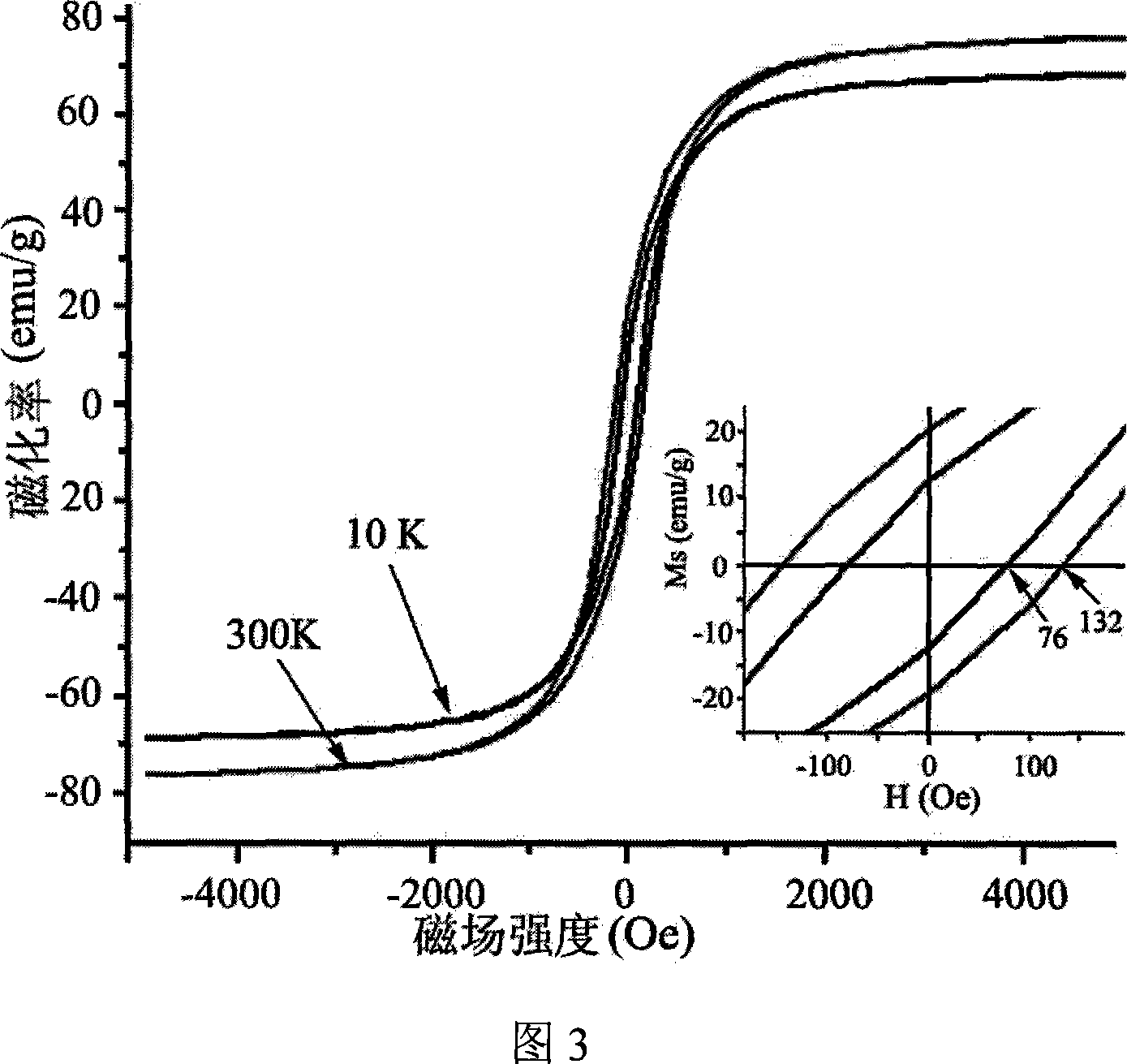
Preparation method for gamma-Fe2O3 magnetic nano particles
A magnetic nanoparticle and nanoparticle technology, applied in the direction of inorganic material magnetism, iron oxide, iron oxide/iron hydroxide, etc., can solve the problems of few reports on magnetic nanoparticles, and achieve good crystallization and dispersion effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] 1.7793 grams of ferrous sulfate (FeSO 4 ·7H 2 O), dissolved in 160 ml deionized water, FeSO 4 The molar concentration is 0.04 mol / liter, and after stirring for 5 minutes, adding 0.7538 gram of concentration is 85% hydrazine hydrate (N 2 h 4 ·H 2 (2), the hydrazine hydrate molar concentration is 0.08 mol / liter, after stirring for 5 minutes, put the above-mentioned prepared solution into the polytetrafluoroethylene lining of the autoclave, the lining volume is 200 milliliters, that is, the filling degree is 80% . The solution was treated at 150°C for 12 hours, and the treated solution was centrifuged to obtain black Fe 3 o 4 nanoparticles, the resulting Fe 3 o 4 is 0.0021 mol. Then the above Fe 3 o 4 The nanoparticles and 1.2092 grams of mass concentration are 30% hydrogen peroxide solution (containing 0.011 moles of hydrogen peroxide) to add the polytetrafluoroethylene lining of the autoclave together, add deionized water to adjust the filling degree to be 80%...
Embodiment 2
[0020] 0.5004 grams of ferrous sulfate (FeSO 4 ·7H 2 O), dissolved in 180 ml deionized water, FeSO 4 Molar concentration 0.01 mol / liter, after stirring for 5 minutes, add 0.72 gram of sodium hydroxide again, sodium hydroxide molar concentration 0.1 mol / liter, stir 5 minutes, finally add 1.0601 gram of mass concentration and be 85% hydrazine hydrate (N 2 h 4 ·H 2 (2), the hydrazine hydrate molar concentration is 0.1 mol / liter, after stirring for 5 minutes, put the above-mentioned prepared solution into the polytetrafluoroethylene lining of the autoclave, the lining volume is 200 milliliters, that is, the filling degree is 90% . The solution was treated at 100°C for 100 hours, and the treated solution was centrifuged to obtain black Fe 3 o4 nanoparticles, the resulting Fe 3 o 4 6x10 -4 Moore. Then the above Fe 3 o 4 Nanoparticles and 2.04 grams of mass concentration are 30% hydrogen peroxide solution (containing 0.018 moles of hydrogen peroxide) to add the polytetrafl...
Embodiment 3
[0022] 33.7977 grams of ferrous chloride (FeCl 2 4H 2 O), dissolved in 170 ml deionized water, FeCl 2 Molar concentration 1.0 mol / liter, after stirring 5 minutes, add 6.8 gram sodium hydroxide again, sodium hydroxide molar concentration 1.0 mol / liter, stir 5 minutes, add 10.012 gram mass concentration at last and be 85% hydrazine hydrate (N 2 h 4 ·H 2 (2), the molar concentration of hydrazine hydrate is 1.0 mol / liter, after stirring for 5 minutes, put the above-mentioned prepared solution into the polytetrafluoroethylene lining of the autoclave, the lining volume is 200 milliliters, that is, the filling degree is 85% . The solution was treated at 200°C for 4 hours, and the treated solution was centrifuged to obtain black Fe 3 o 4 nanoparticles, the resulting Fe 3 o 4 is 0.057 mol. Then the above Fe 3 o 4 The nanoparticles and 6.4599 grams of mass concentration are 30% hydrogen peroxide solution (containing 0.057 moles of hydrogen peroxide) to add the polytetrafluoro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Saturation susceptibility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com