Method for preparing D-amino acid by biological catalysis
A technology of biocatalysis and amino acid, which is applied in the field of compound preparation, can solve the problems of various types of emissions, high price, complex process, etc., and achieve the effect of controlling enantiomeric purity, short route and simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1D
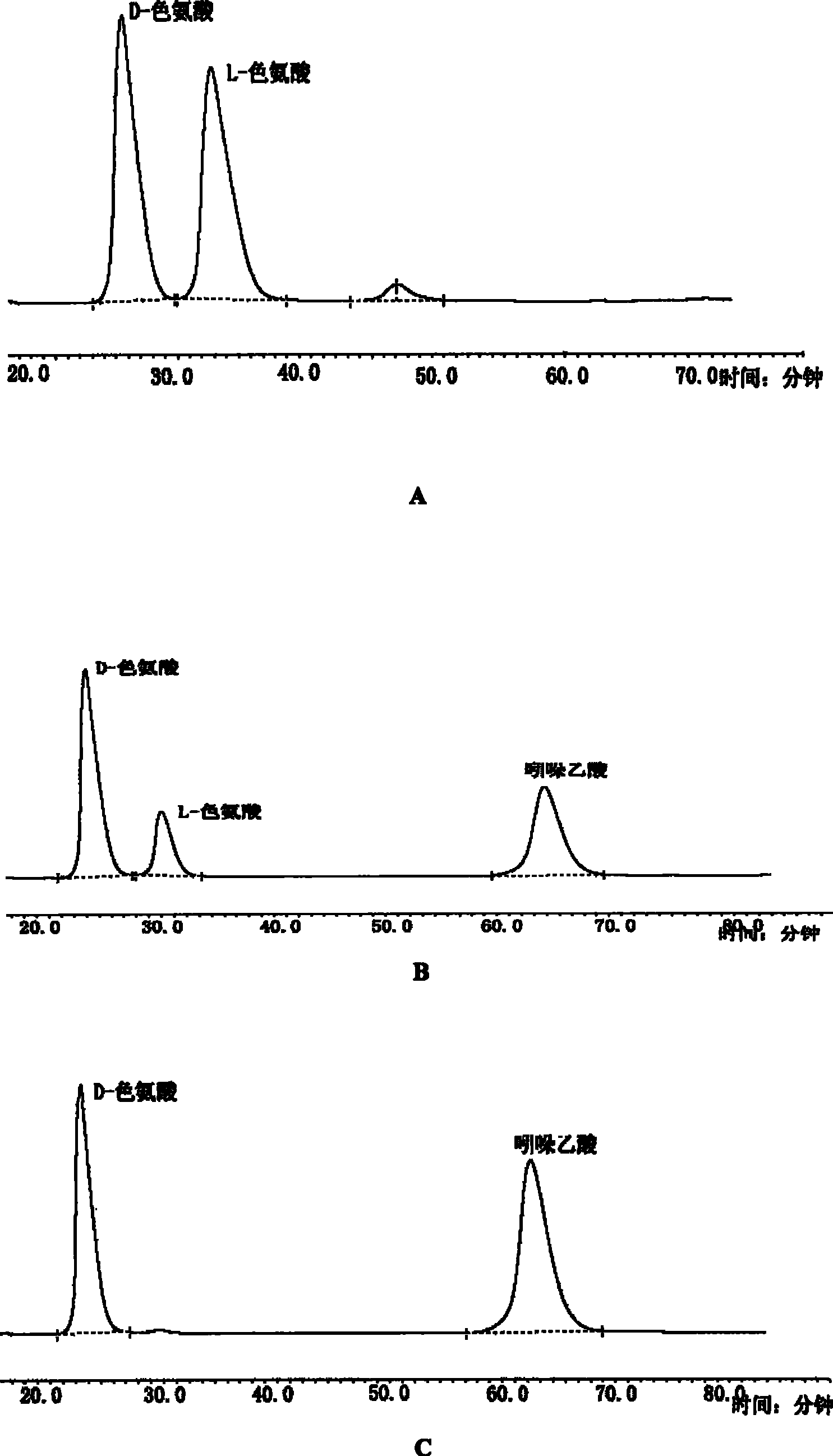
[0019] The preparation of embodiment 1D-tryptophan and indole acetic acid
[0020] In a 500mL shake flask (in 28 flasks at the same time), culture yeast Rhodotorulagraminis (ATCC 20804) (Rhodotorula pasture), grow for 24 hours, centrifuge, pour out the supernatant, freeze, ultrasonically break the wall, and make a homogenate , put it back into the shaker flask, add the same amount of racemate D, 50 mg of L-tryptophan to each bottle; adjust the pH to 7.3, ventilate the air, seal it with ventilation, put it back in the temperature-controlled shaker, shake it circularly, and At 0 hour, 6 hours and 12 hours, one milliliter sample was taken out for analysis in stages, followed by reaction progress, ultrafiltration, dilution, injected into HPLC for follow-up analysis, using Daiselu crown ether column, Crownpak CR(+), D-Try, tr=22min, L-Try, tr=30min. The analysis time was extended to 120 minutes. See results figure 1 , showing the change process of the amount of each substance in ...
Embodiment 2
[0022] The preparation of embodiment 2D-phenylalanine
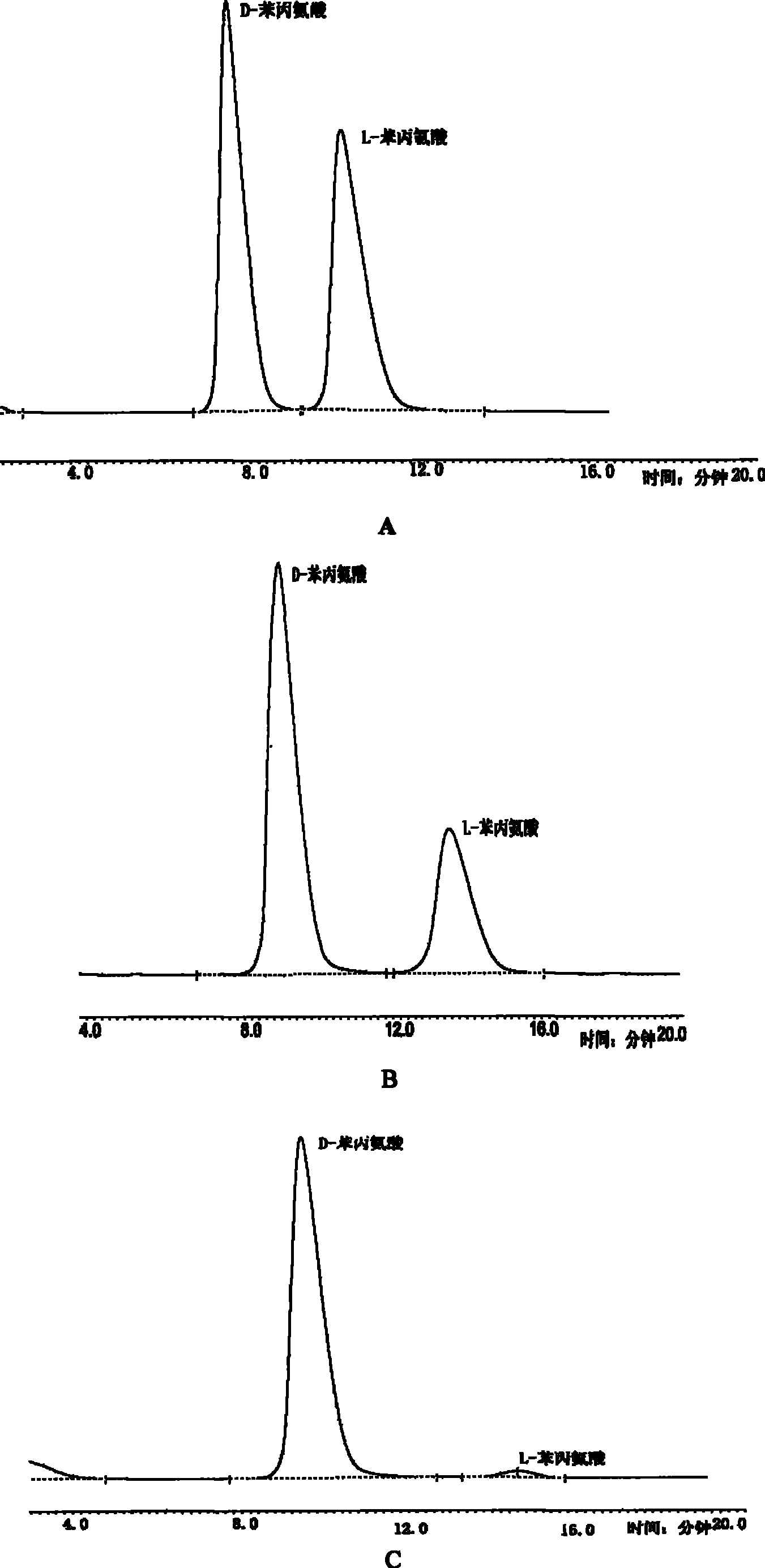
[0023] In a 500mL shake flask, culture yeast Rhodotorula graminis (ATCC 20804) (Rhodotorula grass), grow for 24 hours, centrifuge, pour out the supernatant, freeze, ultrasonically break the wall, make a homogenate, divide evenly and shake back In the bottle, add the same amount of racemate D, L-phenylalanine 50 mg to each bottle; adjust the pH to 7.3, ventilate the air, put it back in the temperature-controlled shaker, shake it circularly, and take out one milliliter of samples for analysis in stages , track the reaction progress, in the reaction of 4h, 6h and 40h, ultrafiltration, dilution, injection into HPLC for tracking analysis, using Daise Crown Ether column, Crownpak CR (+), D-Phe, tr=7.5min, L-Phe , tr=11min. The analysis time was extended to 60 minutes. No other detectable products were found. Separation with ion exchange resin to obtain pure D-phenylalanine (D-Phe).
[0024] see figure 2 , is the change pro...
Embodiment 3D
[0026] The preparation of embodiment 3D-tyrosine
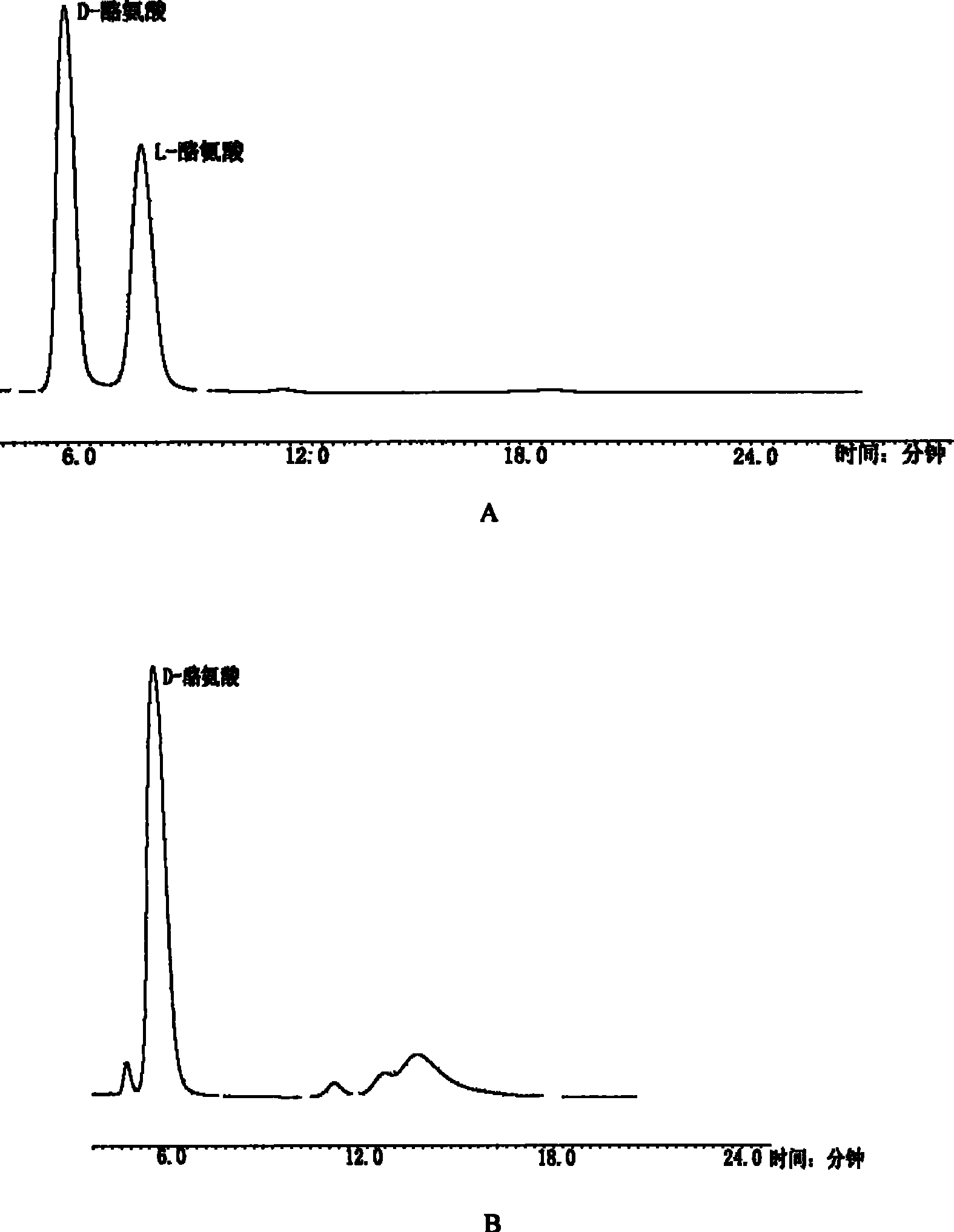
[0027] In a 500mL shake flask, culture yeast (Rhodotorula graminis) (ATCC 20804), grow for 24 hours, centrifuge, pour out the clear liquid, freeze, ultrasonically break the wall, make a homogenate, put it back into the shake flask, Add racemate D, 50 mg of L-tyrosine; adjust the pH to 7.3, ventilate the air, put it back in the temperature-controlled shaker, shake it in a circular manner, take out one milliliter of samples for analysis in stages, and track the reaction progress. After 4 hours of reaction , 6h and 40h, ultrafiltration, dilution, injected into HPLC for follow-up analysis, using a large road crown ether chromatographic column, Crownpak CR (+), D-Tyr, tr=6min, L-Tyr, tr=9min. The analysis time is extended to 60 minutes. Separation with ion exchange resin to obtain pure D-tyrosine (D-Tyr).
[0028] see image 3 , is the change process of the amount of each substance in the biotransformation process of tyrosine. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com