Compositions and methods for regulated protein expression in gut
A digestive tract and protein technology, applied in animal/human protein, fusion cells, pharmaceutical formulations, etc., can solve problems such as desensitization of the body's insulin response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0160] This example describes the establishment of gut endocrine cell lines for the study of regulation of insulin production and in vivo targeting of insulin expression. This example also describes the construction of a human insulin gene expression vector.
[0161] Establish GIP expressing cell line to study whether GIP promoter is effective for K cells to express insulin gene. This cell line was cloned from the mouse intestinal cell line STC-1, a mixed enteroendocrine cell population (Rindi et al., Am. J. Pathol. 136:1349 (1990)). K cells in mixed cells can be visually identified by transfection of a green fluorescent protein expression plasmid driven by the approximately 2.5 Kb rat GIP promoter. The rat GIP promoter was obtained from the rat genome lambda DASH library (Strategene) by plaque hybridization with rat GIP cDNA clone plaques as previously described (Boylan et al., J. Biol. Chem. 273: 17438 (1997)), and It was subcloned into the promoterless pEGFP-1 plasmid (Cl...
Embodiment II
[0171] This example describes transgenic mice that produce insulin in response to glucose.
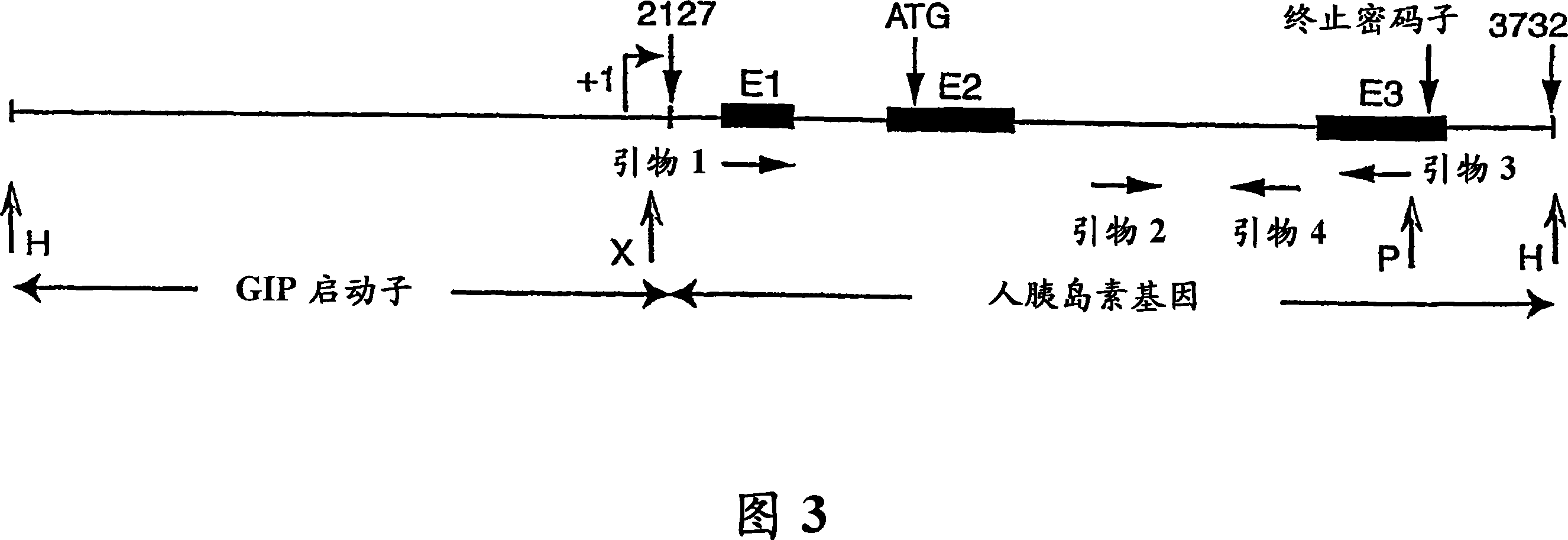
[0172] Using the human insulin expression construct GIP / Ins described in Example 1, the GIP / insulin fragment (about 4.1 Kb) was digested with HindIII. Transgenic mice were generated by microinjecting approximately 4.1 Kb transgenic pronuclei into fertilized eggs and implanting the fertilized eggs into surrogate female mice. Transgenic offspring were identified by Southern blot analysis. The otic DNA (Figure 3) was digested with XhoI and PvuII, separated by electrophoresis and transferred to a nylon membrane. To detect the transgene, primers 2 and 4 were used to amplify a 416 bp human insulin gene fragment containing intron 2 (Figure 3). By using [α- 32 P]dCTP was randomly labeled to prepare PCR products as probes, and the bands were detected by autoradiography. DNA analysis results were further confirmed by PCR amplification of genomic DNA using primers 2 and 4. The positive mice ...
Embodiment III
[0179] This example shows that insulin production in transgenic mice provides normal glucose homeostasis and protects against developing diabetes. Human insulin production by gut K cells in transgenic mice was also regulated by diet. This example also describes data showing that glucose-inducible insulin production in transgenic mice provides glucose homeostasis following pancreatic β-cell destruction.
[0180] Plasma human insulin levels in transgenic mice were analyzed in response to food intake. Briefly, plasma insulin levels were measured using a human-specific insulin ELISA kit (ALPCO) following the supplier's instructions. The assay has <0.01% cross-reactivity with human proinsulin and C-peptide and cannot detect mouse insulin. Plasma C-peptide detection was performed with a rat / mouse C-peptide RIA kit (Linco). This assay showed no cross-reactivity with human C-peptide.
[0181] In pooled plasma samples collected after oral glucose administration, insulin in transgen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com