3-deoxyglucosone and skin
A technology of deoxyglucosone and skin, applied in the field of 3-deoxyglucosone and skin, which can solve the problems of harmful activity, no effective therapeutic agent and/or diagnostic agent, increased concentration, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0468] The present invention includes methods for the preparation and use of pharmaceutical compositions comprising compounds for the treatment of various skin-related diseases, disorders or conditions described herein, including skin aging, Photoaging and wrinkling of the skin. The invention also includes non-skin diseases and disorders associated with 3DG, including, but not limited to, gum diseases and disorders. Such pharmaceutical compositions may consist of a single active ingredient in a form suitable for administration to a subject; or the pharmaceutical composition may comprise at least one active ingredient and one or more pharmaceutically acceptable carriers , one or more other components, or some combination thereof. The active ingredient may be present in the pharmaceutical composition in the form of a physiologically acceptable ester or salt, such as in combination with a physiologically acceptable cation or anion, as is well known in the art.
[0469] The barr...
Embodiment 1
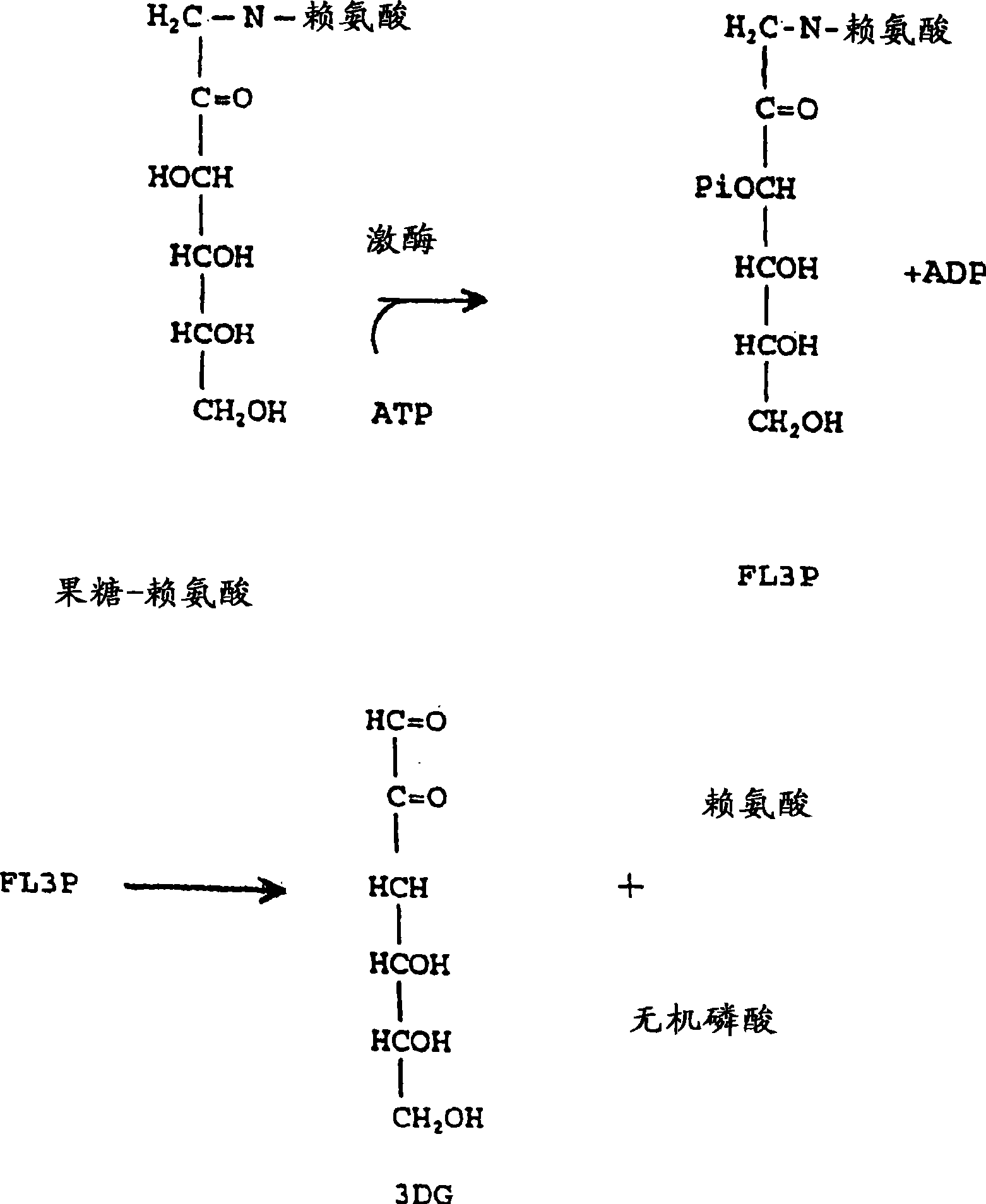
[0527] Isolation and identification of FL3P:
[0528] The following experiments were performed to verify that fructose-lysine (FL), such as FL3P, could be identified in its phosphorylation state. Perchloric acid extracts of diabetic rat kidney 31 P NMR analysis, at 6.24 ppm revealed a new sugar-monophosphate resonance that was not observed in non-kidney tissues and whose levels were significantly reduced in non-diabetic kidneys. The compound responsible for the observed resonance was isolated by chromatography of the extract on a microcrystalline cellulose column using 1-butanol-acetic acid-water (5:2:3) as eluent. The structure was determined to be fructose-lysine 3-phosphate by proton 2D COZY. This result was confirmed hereinafter by injecting animals with FL prepared as described above (Finot and Mauson, 1969, Helv. Chim. Acta, 52:1488) and showing direct phosphorylation to FL3P.
[0529] Phosphoric acid was confirmed to be at the carbon-3 position using FL deuterated ...
Embodiment 2
[0531] Synthesis of FL3P:
[0532] 1 mmol of dibenzyl-glucose 3-phosphate and 0.25 mmol of α-carbocarboxylate-lysine were refluxed in 50 ml of MeOH for 3 hours. The solution was diluted with 100 ml of water and chromatographed in the pyridinium form on a Dow-50 column (2.5 x 20 cm), eluting first with water (200 ml) and then with 600 ml of buffer (0.1M pyridine and 0.3M acetic acid). Compounds of interest were eluted at the end of the water wash and at the beginning of the buffer wash. The results confirmed that FL3P was obtained in 6% yield after removal of cbz and benzyl blocking group using 5% Pd / C under 20 ps hydrogen.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com