A plurality of reconstructing antibiotic peptide and preparation method and application thereof
An antibacterial peptide and gram-positive bacteria technology, applied in the field of modified antibacterial peptides and their preparation, can solve problems such as loss of binding ability, easy to cause hemolysis, etc., achieve easy preparation, enhance bactericidal power and LPS binding power, reduce Effects of side effects such as hemolysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
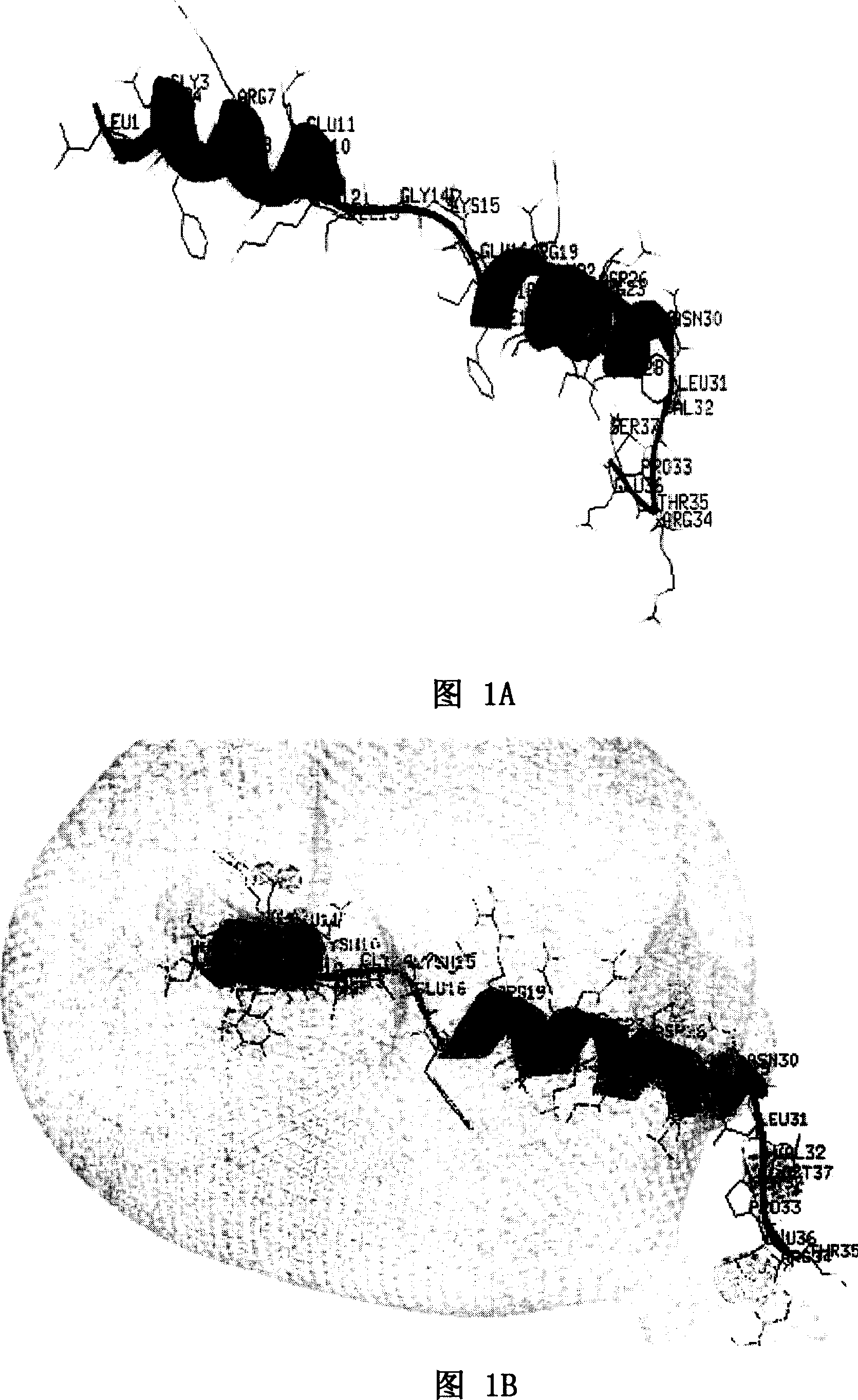
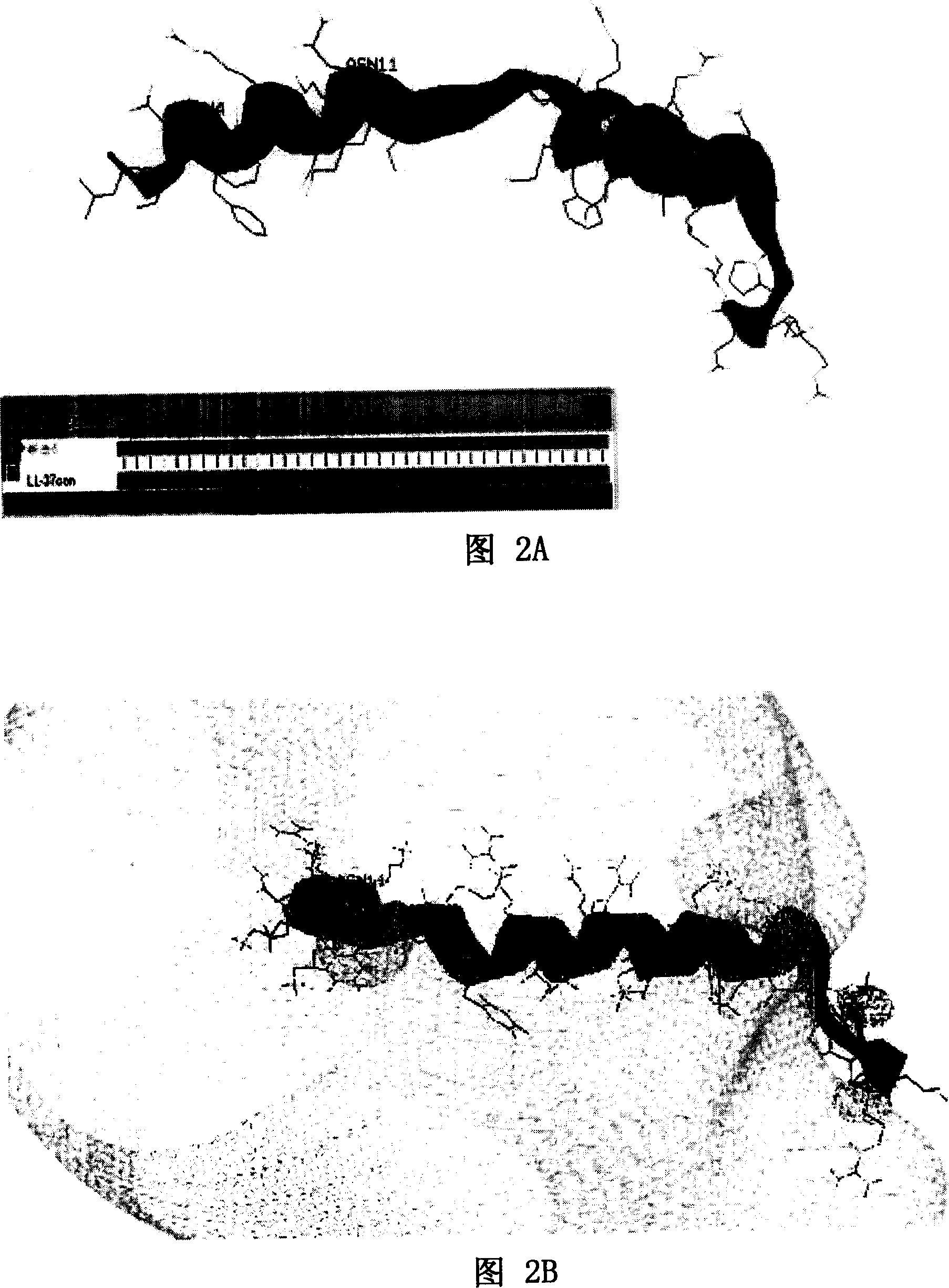
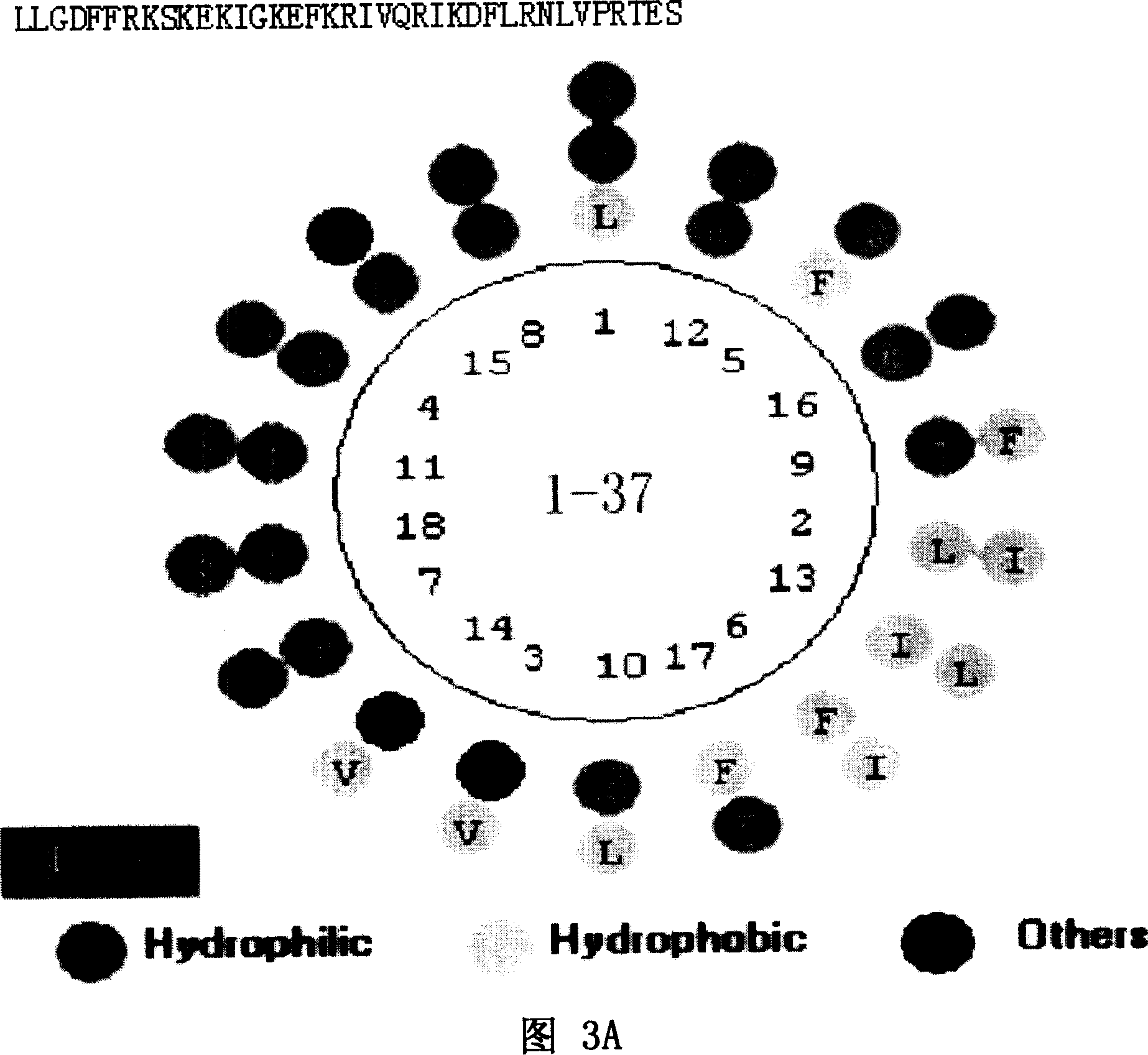
[0058] As shown in Figure 1A and Figure 1B, Figure 1A is a spatial helical structure diagram of natural LL-37, and Figure 1B is a charge distribution diagram of natural LL-37. The three-dimensional structure of NMR of natural LL-37 is detected, and the spatial structure of LL-37 can be obtained, and the position of each amino acid residue and each atom in LL-37 in space, the connecting bonds between atoms, Angle, length and other data can accurately obtain the spatial helical structure of LL-37 and its charge distribution diagram. Peptide rLL-37, namely rLL-37-1~rLL-37-17, rLL-24-1~rLL-24-8, rLL-18-1~rLL-18-1, rLL-30-1~rLL- 30-10. The sequence listing is as follows:
[0059] (1) Reconstructed 37-peptide antibacterial peptide sequence (rLL-37):
[0060] rLL-37-1:
[0061] Leu Leu Gly Asn Phe Phe Arg Lys Ser Lys Asn Lys Ile Gly Lys Glu Phe Lys Arg Ile Val Gln Arg
[0062] Ile Lvs Asp Phe Leu Arg Asn Leu Val Pro Arg Thr Glu Ser
[0063] rLL-37-2:
[0064] Leu Leu Gly Asn P...
Embodiment 2
[0179] In order to prepare the rebuilt rLL-37 peptide antimicrobial peptide, the present invention also synthesized a set of negatively charged carrier protein sequence (Carrier protein molecule, CPM), which is based on the desired protein sequence. Designed for negative charge and high water solubility, it was detected on Gene Bank and has no identical gene sequence.
[0180] (1) The gene sequence of the stored protein is:
[0181] GAA GTT TGG AAC GCA CTT GAT GCA CTG GAG CTG GTA ATCCAA CAA GAG GAG GGT TCT AAT GGT ACT TCT ACT GGA TCC GAGGGC
[0182] (2) The amino acid sequence of the stored protein is:
[0183] Glu Val Trp Asn Ala Leu Asp Ala Leu Glu Leu Val Ile Gln Gln Glu Glu Glu Ser Asn Gly Thr Ser Thr Gly Ser Glu Gly
[0184] (3) The charge carrying the protein is: pHi=2.7; at pH7.4, the charge is -6.0
Embodiment 3
[0186] Preparation of 24-peptide series antimicrobial peptides and 18-peptide series antimicrobial peptides:
[0187] The antibacterial peptide sequence is relatively short, although it has a strong positive charge, by appropriately increasing the pH value of the synthesis solution to 8.35, the charge on the short peptide can be appropriately reduced, so that it can be directly synthesized by solid-phase chemical method.
[0188] (1) Preparation of rLL-24-1:
[0189] Synthesis of rLL-24-1 polypeptide by solid-phase chemical synthesis was carried out on an ABI 431A peptide synthesizer produced by PE Company in the United States. The standard Fmoc protocol method was used to couple arginine twice.
[0190] First, 0.25 mmol of HMP resin (hydroxymethylphenoxymethyl polystyrene resin, produced by PE Company) was selected, and the peptide chain was extended from the carboxyl terminal to the amino terminal one by one according to the polypeptide sequence.
[0191] After the synthes...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com