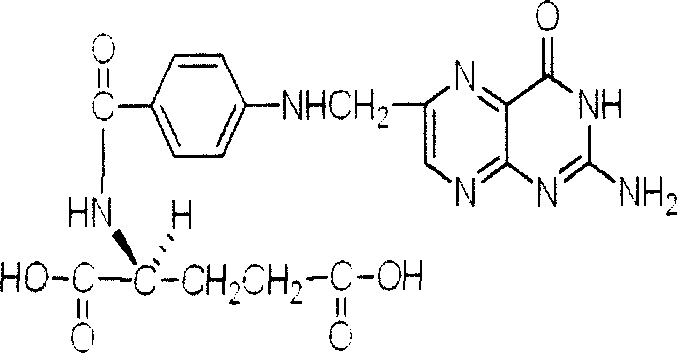
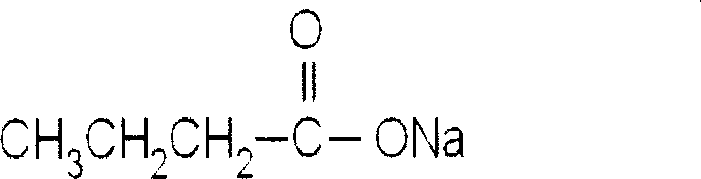Medicinal composition for preventing and curing stomach and intestine tumor through epigenetic modification
A technology of genetic modification and composition, applied in drug combination, anti-tumor drugs, drug delivery, etc., can solve the problems of limited single oncogene or tumor suppressor gene methylation or acetylation research, and achieve reversal of gastrointestinal tract. Mucous membrane atrophy, small effect of tumor prevention and treatment, effect of improving anti-tumor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Colorectal cancer cell lines SW1116, Colo-320 and gastric cancer cell line MKN45 were routinely cultured in RPMI1640 medium. Sodium butyrate (NaBu 1×PBS dilution, 550mg / L), folic acid (FA2mg / L), and the drug group of the present invention (containing NaBu 550mg / L, FA2mg / L) were treated for 24 h. The control group was without any treatment group. After synchronization treatment, the cell cycle was detected by flow cytometry, and the tumor suppressor gene p21 was detected by RT-PCR WAF1 mRNA expression, p21 analysis by co-immunoprecipitation WAF1 Levels of acetylated histones H3 and H4 in gene-associated chromatin. Results: Compared with the control group, the apoptosis index of MKN45 cells treated with FA increased, but had no significant effect on the cell cycle. NaBu group can up-regulate p21 by increasing the acetylation level of SW1116 and Colo-320 cells WAF1 Expression, induces aggregation of acetylated histones H3 and H4. After 24 hours in the drug group of the...
Embodiment 2
[0037] 1. Preparation of colorectal cancer animal model
[0038] Purchasing inbred ICR clean-grade female mice weighing 18-20 g from the Chinese Academy of Sciences in Shanghai, carcinogen dimethylhydrazine (DMH), white powdery crystals. Induce a mouse colorectal cancer model, and intervene with FA, NaBu, FA and NaBu pharmaceutical composition during the modeling process.
[0039] 2. Grouping of mice and drug intervention
[0040] 1) Blank control group
[0041] 2) FA control group
[0042] 3) NaBu control group
[0043] 4) Pharmaceutical composition control group
[0044] 5) DMH group: DMH neck was subcutaneously injected into the model, and 0.4% solution was prepared with sterile normal saline before each injection, and NaHCO 3 Adjust its pH to 6.5-7.0, and inject 20 mg / kg subcutaneously in the neck once a week for 20 consecutive weeks.
[0045] 6) DMH+FA: FA was dissolved in drinking water of mice at a concentration of 0.02%; continuous for 20 weeks.
[0046] 7) DMH+Na...
Embodiment 3
[0064] In the trial study of clinical patients, patients with gastrointestinal mucosal atrophy were treated with the drug of the present invention, followed up to monitor the patient's serum folic acid concentration and the methylation level of related genes, and regularly carried out pathological examination on the mucosal tissue. Preliminary observations found that After being intervened by the medicine of the present invention, the symptoms of the gastrointestinal tract of the patient are relieved, the atrophy of the mucosa can be improved, and some dysplasia can be reversed, and no obvious toxic and side effects are found.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com