TRAIL receptor I and/or TRAIL receptor 2 specific antibody and its use
An antibody and receptor technology, applied in the field of unique monoclonal antibodies, which can solve the problems of lack of receptor selectivity, short in vivo half-life of recombinant TRAIL protein, weakening, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0200] Example 1: Preparation of TRAIL-R1 and TRAIL-R2 mixed dimers
[0201] 1. Cloning of TRAIL-R1 and TRAIL-R2 cDNA by RT-PCR
[0202] a) Prepare the template
[0203] Total RNA was extracted from human cervical cancer Hela cells using TRIZOL reagent (GibcoBRL). Then, using the total RNA as a template, cDNA was synthesized by reverse transcription, and the Promega cDNA reverse transcription finished kit was used to operate according to its instruction manual.
[0204] b) Synthetic PCR oligonucleotide primers:
[0205] TRAIL-R2 5' end primer: 5'-gacgatgcccgatctactttaaggg-3'
[0206] TRAIL-R2 3' end primer: 5'-ccactgggtgatgttggatggg-3'
[0207] TRAIL-R1 5' end primer: 5'-gacgatgcccgatctactttaaggg-3'
[0208] TRAIL-R1 3' end primer: 5'-gacgatgcccgatctactttaaggg-3'
[0209] c) PCR reaction:
[0210] Take 5 microliters of template cDNA, add 5 microliters of 5' and 3' primers, 10 microliters of 10-fold concentrated PCR buffer, 4 microliters of dNTP, and 5 units of TaqPCR po...
Embodiment 2
[0223] Example 2: Preparation of anti-human TRAIL-R1 and TRAIL-R2 monoclonal antibodies
[0224] 1. Immunization of mice
[0225] The TRAIL-R1 and TRAIL-R2 fusion proteins purified by affinity chromatography were mixed with Freund's complete adjuvant 1:1, and 0.2 ml protein adjuvant mixture was injected into the paws of 2 Balb / c mice (female, week-old : 6 to 8 weeks). Ten days later, 0.2 ml of the mixture of protein and Freund's incomplete adjuvant was injected again. One week later, 0.2 mg of purified protein was injected again. Once a week, repeat 3 times. Three days after the last injection, the abscess and inguinal lymph nodes of the mice were taken for the preparation of hybridomas.
[0226] 2. Cell Fusion
[0227] A single cell suspension was made from the fossa and inguinal lymph nodes, and mixed with NS1 myeloma cells at a ratio of 2:1. Wash three times with serum-free RPMI-1640 medium. Add 1 ml of 37°C preheated PEG1500, and gently stir the mixture of PEG1500 a...
Embodiment 3
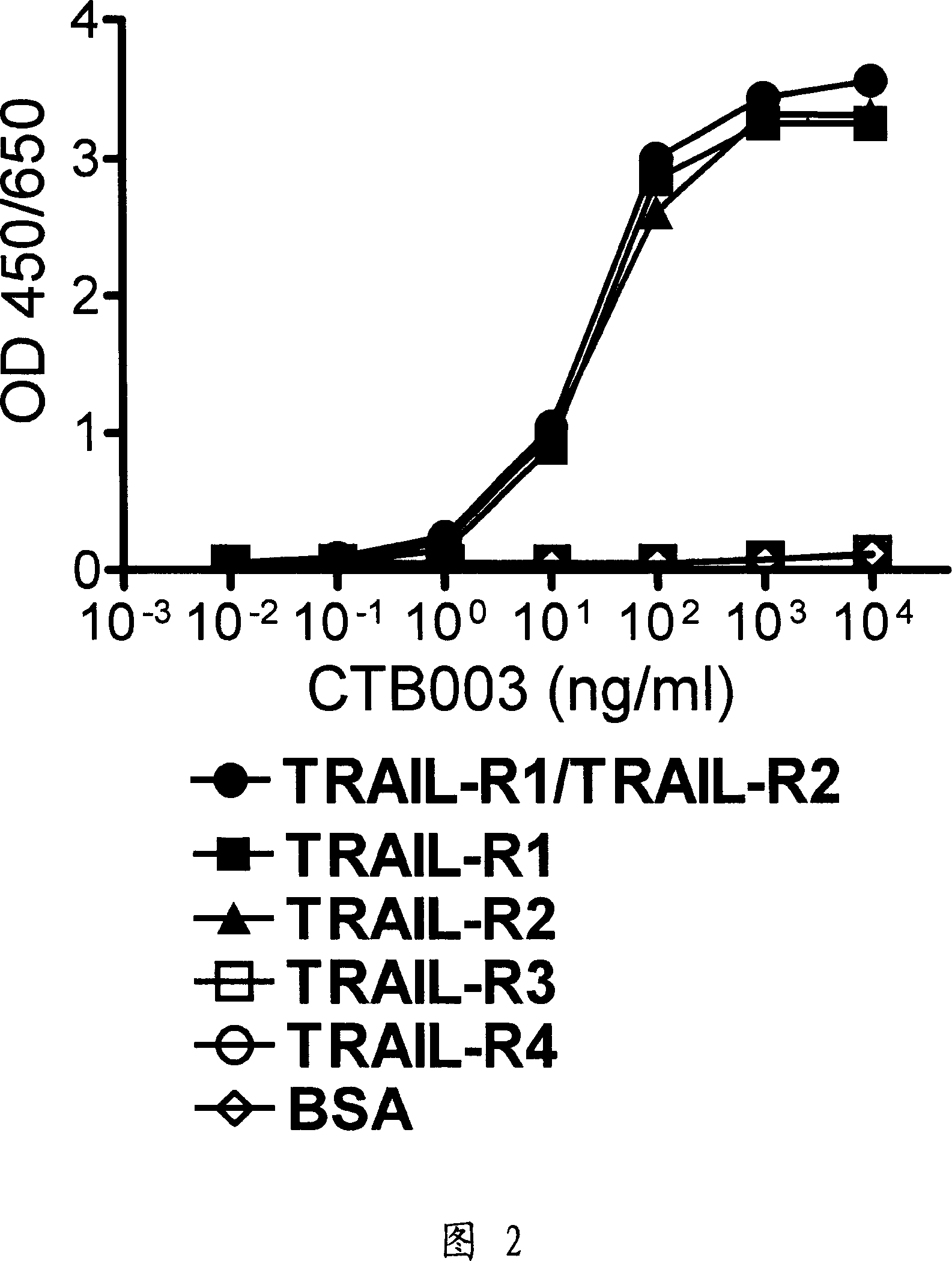
[0241] Example 3: TRAIL receptor binding specificity of CTB003
[0242] Since the five TRAIL receptors have high amino acid sequence homology in their extracellular regions, we further tested the reactivity of CTB003 to other TRAIL receptors. TRAIL-R1, TRAIL-R2, TRAIL-R3 and TRAIL-R4 were respectively coated onto microtiter plates, and then blocked with PBS buffer containing 3% BSA. After blocking for 1 hour, different concentrations of purified CTB003 were added. After incubation at 37°C for 1 hour, horseradish peroxidase-labeled goat anti-mouse IgG was added, followed by TMB substrate buffer 30 minutes later. After ten minutes, the reaction was quenched with 2 equivalents of sulfuric acid. Then mark it with a microplate reader.
[0243] The results showed that CTB003 showed an equivalent and dose-related antigen-binding response to TRAIL-R1 and TRAIL-R2. Meanwhile, CTB003 did not exhibit antigen-binding responses to TRAIL-R3 and TRAIL-R4 within the range of antibody conc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com