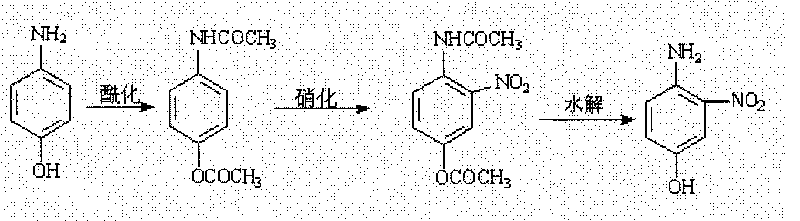
Process of preparing 4-amino-3-nitro phenol
A technology for nitrophenol and p-aminophenol, which is applied in the field of fine chemical intermediate synthesis, can solve the problems of azide compounds such as explosion danger, low yield, difficulty in selective reduction, etc., and achieves high acylation activity, cost reduction, The effect of reducing the amount of production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0018] In a 250 ml four-neck flask equipped with a thermometer and a stirring device, add 21.0 grams (0.1927 mol) of p-aminophenol, 40 grams (0.3854 mol) of acetic anhydride (refined anhydrous) and a small amount of glacial acetic acid, and heat to reflux at 128 ° C. After reacting for about 2 hours, the reaction solution was cooled to 25° C., then 30.0 ml (0.7142 mol) of 98% nitric acid was added dropwise and the temperature was kept constant, and stirred for 1.0 hour under strong acid conditions. Then 20 milliliters (0.4444 mol) of 65% nitric acid was added, the temperature was controlled at 20° C., and the reaction was stirred for 1.5 hours. Then the reaction solution was poured into ice water to precipitate yellow crystals, which were washed 2 to 3 times with water until neutral, filtered with suction, and the filter cake was dried to obtain yellow 2-acetamido-5-acetoxynitro 34.6 grams of benzene, the yield is 75.5%. Melting point: 144℃~146℃.
example 2
[0020] In a 250 ml four-necked flask equipped with a thermometer and a stirring device, add 21.0 grams (0.1927 mol) of p-aminophenol, 59 grams (0.5781 mol) of acetic anhydride (refined anhydrous) and a small amount of glacial acetic acid, and heat to reflux at 128 ° C. After reacting for about 2 hours, the reaction liquid was cooled to 25° C., then 40.0 ml (0.9541 mol) of 98% nitric acid was added dropwise and the temperature was kept constant, and stirred for 1.0 hour under strong acid condition. Then 22 milliliters (0.4889 mol) of 65% nitric acid was added, the temperature was controlled at 20° C., and the reaction was stirred for 1.0 hour. Then the reaction solution was poured into ice water to precipitate yellow crystals, which were washed 2 to 3 times with water until neutral, filtered with suction, and the filter cake was dried to obtain yellow 2-acetamido-5-acetoxynitro 32.8 grams of benzene, the yield is 71.6%. Melting point: 144℃~146℃.
[0021] Synthesis of 4-amino-...
example 3
[0023] Add 5.4 (0.0227mol) gram of 2-acetamido-5-acetoxynitrobenzene prepared in Example 1 and 30.0 milliliters of 3mol / L NaOH solution in a 150 milliliter four-necked flask with a thermometer and a stirring device, Heat to 60°C and control the stirring reaction at 50-55°C for 4 hours. After the reaction is finished, the reaction solution is cooled to below 10°C, and then the pH value of the reaction solution is adjusted to 3-4 with a hydrochloric acid solution with a mass ratio of 1:1 to water. Red crystals precipitated. Suction filtration, drying the filter cake to obtain 2.7 g of brown-red 4-amino-3-nitrophenol. The yield was 77.1%. Melting point: 149℃~150℃.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com