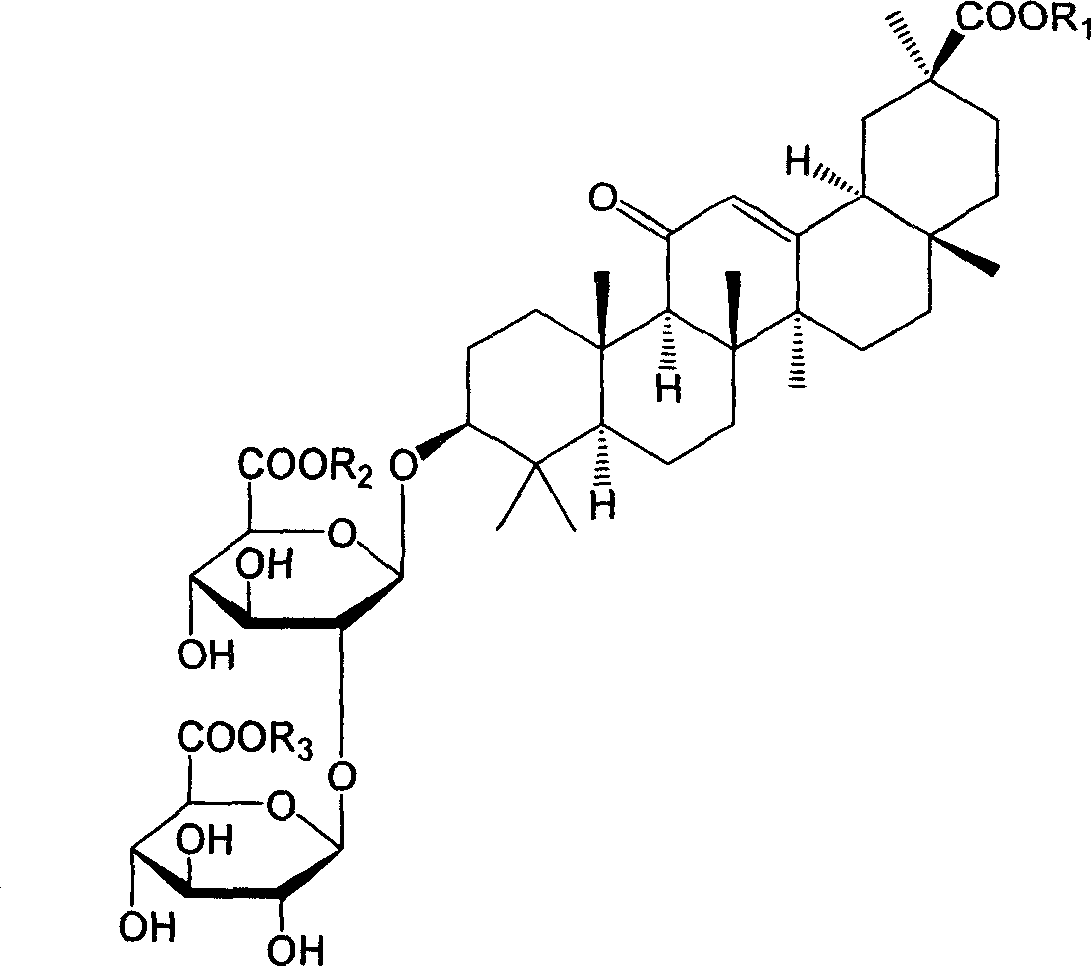
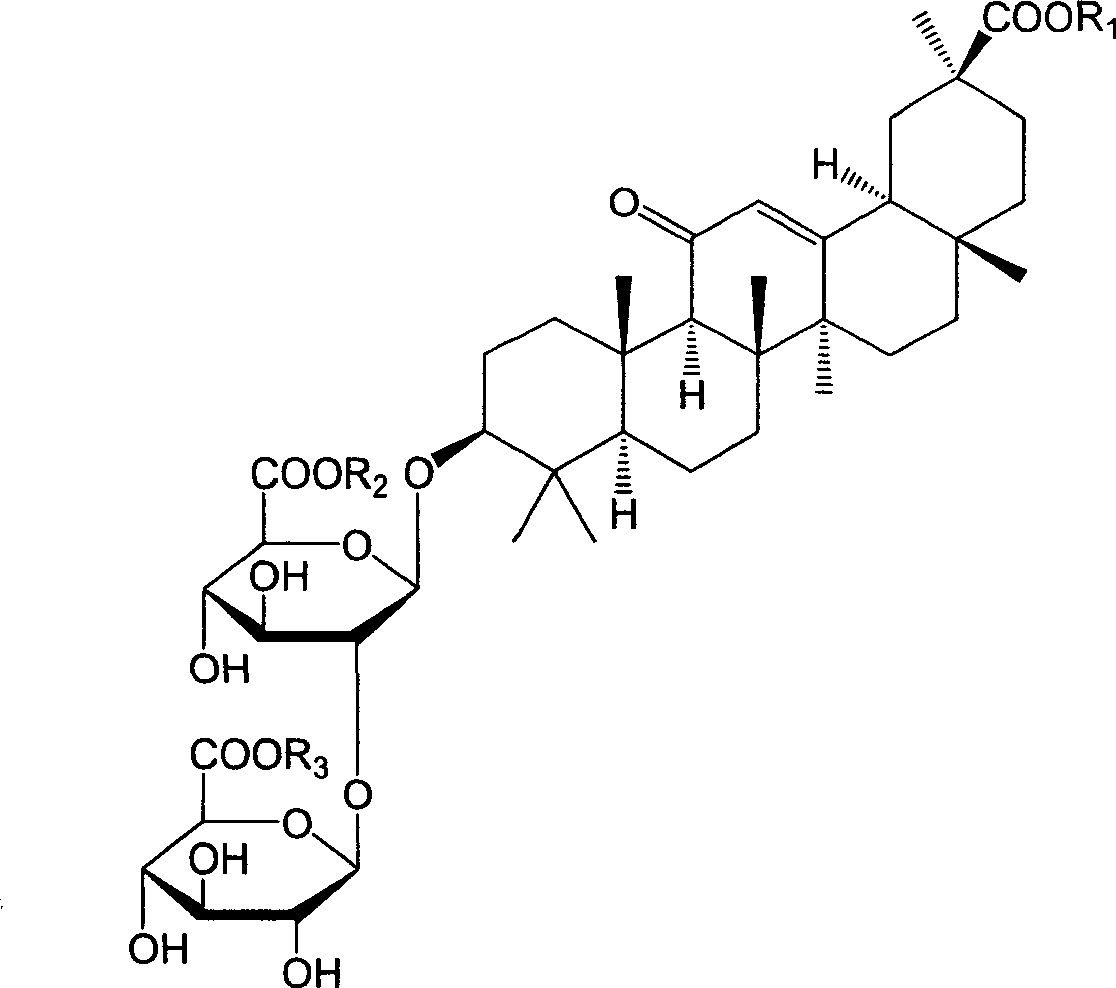
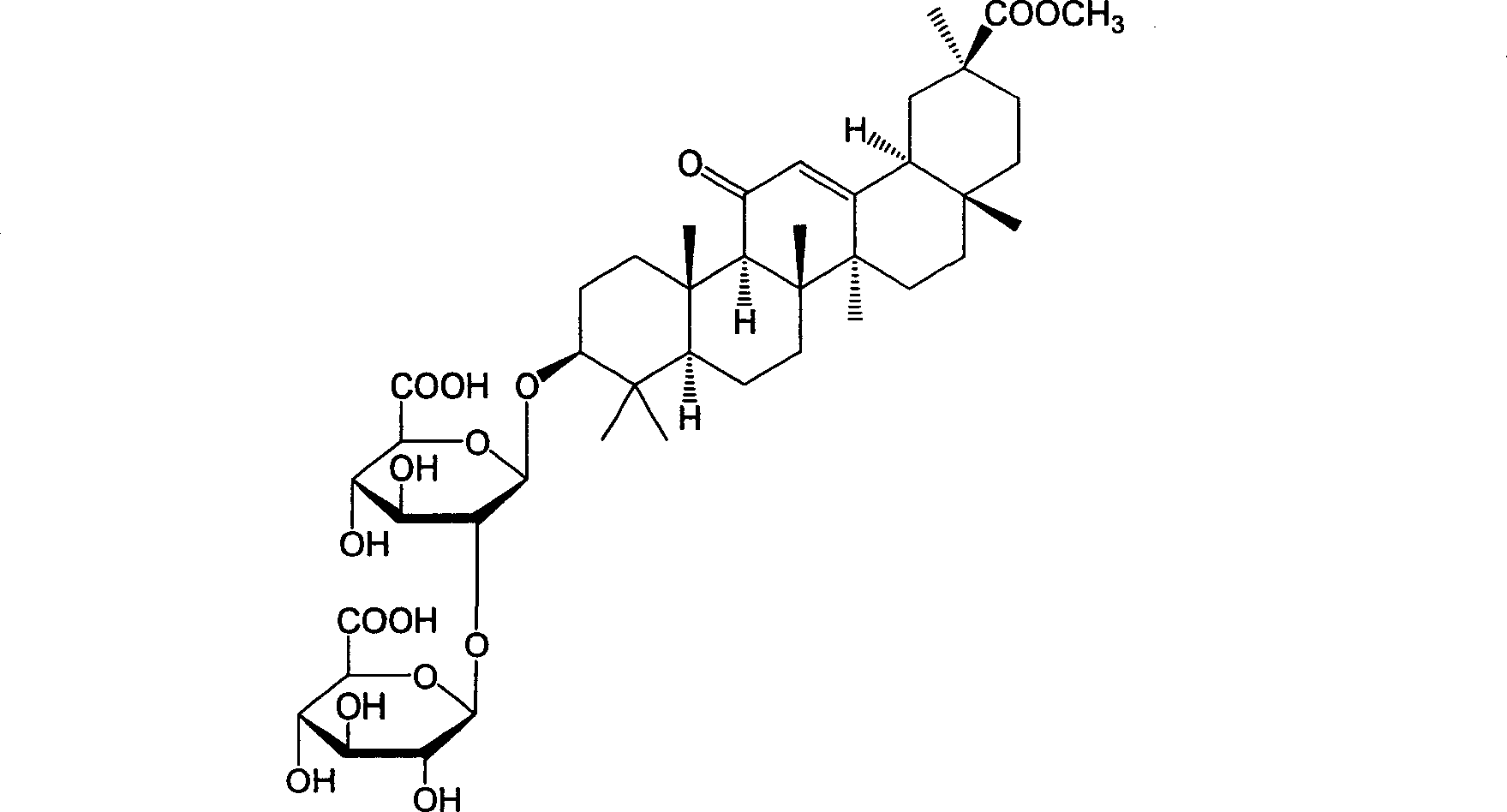
18alpha- liquorice acid derivatives and preparation thereof
A technology of derivatives and glycyrrhizic acid esters, applied in the field of glycyrrhizic acid derivatives and their preparation, can solve the problems of low oral bioavailability and inconvenience to patients, and achieve good oral resistance to liver damage, excellent lipophilicity, and excellent bioavailability. degree of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: Preparation of 18α-glycyrrhizic acid
[0044] Add 500 g of glycyrrhizic acid to 5 liters of 1M NaOH aqueous solution, heat to 70°C and stir for 5 hours, adjust the pH value to 2-4 after cooling, filter, wash with water, and dry to obtain 382 g of 18α-glycyrrhizic acid with a yield of 76.4%.
Embodiment 2
[0045] Example 2: 18α, 20β-methoxyacyl-11-oxo-n-oleanane-12-en-3β-yl-2-O-β-D-glucopyranuronalcarboxyl-α-D-glucopyranosyl Preparation of furanuronic acid
[0046]
[0047] Dissolve 10g of 18α-glycyrrhizic acid in 100ml of methanol, slowly add 3ml of acetyl chloride dropwise at room temperature (about 0.5h), continue to stir for 1h after addition, filter, and dry in vacuo to obtain the target compound, namely methyl 18α-glycyrrhizinate 6.0 grams, with a melting point of 244-247°C.
Embodiment 3
[0048] Example 3: 18α, 20β-ethoxyacyl-11-oxo-n-oleanane-12-en-3β-yl-2-O-β-D-glucopyranuronalcarboxyl-α-D-glucopyranosyl Preparation of furanuronic acid
[0049]
[0050] Dissolve 10g of 18α-glycyrrhizic acid in 100ml of ethanol, slowly add 3ml of acetyl chloride dropwise at room temperature (about 1h), continue stirring for 5h after addition, filter, and dry in vacuo to obtain the target compound, 7.3g of ethyl 18α-glycyrrhizinate , melting point 235 ~ 238 ℃.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com