Toxicity T cell position vaccine of the cell for treating Hepatitis B and the preparing method
A technology for cytotoxicity and hepatitis B, applied in antiviral agents, pharmaceutical formulations, antibody medical components, etc., can solve the problems of incomplete elimination of viruses and poor long-term curative effect, and achieve the effect of enhancing cellular immune response and promoting activation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Expression of T cell epitope fusion protein
[0037] 1. Select CTL epitope
[0038] From HBV antigen CTL epitope data, 21 CTL epitopes and two general Th cell epitopes covering human leukocyte antigen (HLA) HLAA2, A3, B7 supertype and A2402 phenotype were selected.
[0039] The following are the CTL epitopes in the different HBV antigens selected in the present invention (the numbers indicate the amino acid position of the epitope corresponding to the HBV antigen, and the amino acid sequence and HLA restriction of the epitope are in parentheses.):
[0040] HBV pre-S2 antigen: preS2 109-123 (MQWNSTALHQALQDP, A3), preS2 152-161 (SILSKTGDPV, A3)
[0041] Surface antigen: S 177-185 (VLQAGFFLL, A2), S 183-191 (FLLTRILTI, A2), S 249-257 (LLCLIFLLV, A2), S 313-321 (IPIPSSWAF, B7), S 335-343 (WLSLLVPFV, A2), S 348-357 (GLSPTVWLSV, A2)
[0042] Core antigen: C 18-27 (FLPSDFFPSV, A2 / B7 / B51), C 117-125 (EYLVSFGVW, A2402), C 141-151 (STLPETTVVRR, A3)
[0043] Polymerase anti...
Embodiment 2
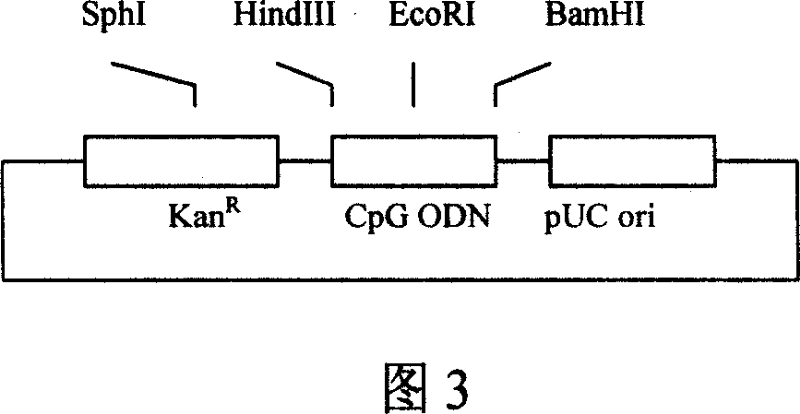
[0091] Example 2: Construction of immune adjuvant plasmid
[0092] 1. Amplification of CpG ODN vector DNA fragment
[0093] Using the eukaryotic expression plasmid vector pVAX1 (Invitrogen product) as a template, PCR amplifies the DNA fragments encoding the kanamycin resistance gene (KanR) and the pUC plasmid replication origin (pUC ori),
[0094] Upstream primer: 5'-GAATTCAAGCTTAGAGACAGGATGAGGATC-3',
[0095] Downstream primer: 5'-GAATTCGGATCC GTCAACGCGT ATATCTGG-3'.
[0096] The reaction conditions and procedures are the same as before.
[0097] 2. Connection of CpG ODN vector plasmid pKO
[0098] The amplified product obtained above was digested with endonuclease EcoRI, and the obtained DNA fragment was subjected to agarose gel electrophoresis, the target DNA band was recovered, and then self-circularized and ligated with T4 DNA ligase, and the ligated product was transformed into E. coli The resulting recombinant plasmid pKO. pKO contains KanR and pUC ori, and three single re...
Embodiment 3
[0108] Example 3: Preparation of vaccine
[0109] Purify the T cell epitope fusion antigen from yeast cells by hydrophobic chromatography and molecular sieve methods. Purify the plasmid adjuvant from E. coli with a plasmid mass preparation system (product of Qiagen, Germany), and dissolve it in a certain buffer, such as phosphate buffer. Solution (pH 7.0~7.4), with certain compatibility and certain concentration, such as 40~80μg of T cell epitope fusion antigen and 100~500μg of plasmid adjuvant. Mix the two to be a candidate vaccine for animal testing. Detect its efficacy, pharmacological activity and possible side effects, analyze its immunogenicity, and evaluate its application prospects as a therapeutic hepatitis B vaccine.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com