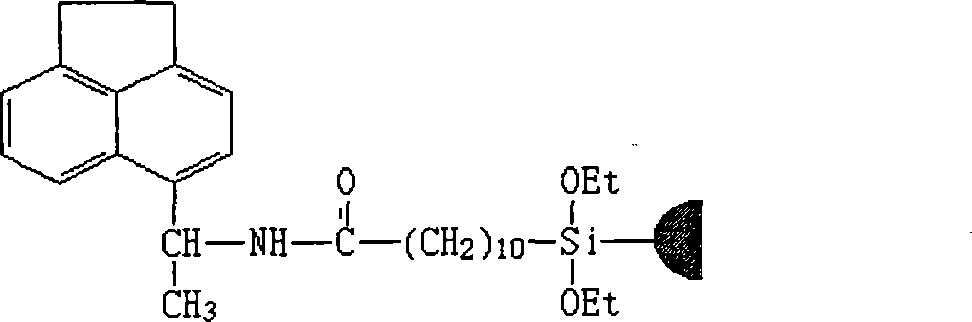2-nitro-benzoyl-imino-acenaphthylene derivative compound and use thereof
A technology of dinitrobenzoyl imino acenaphthene and compound is applied in the field of new dinitrobenzoyl imino acenaphthene derivative compounds, which can solve the problem that the spatial structure is not good enough, cannot be split, and can be split. Not good and other problems, to achieve the effect of easy control of synthesis conditions, low cost and mature synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] This example is the preparation of 5-[1-(3,5-dinitrobenzoimide)-4-pentenyl]-acenaphthene compound and its chiral substance.
[0035]Synthesis of 5-acetylacenaphthylene (2) Take 12.0g of anhydrous aluminum trichloride and dissolve it in 120ml of dichloromethane, add 4.4g of acetyl chloride while stirring in an ice bath, and finally add 10.4g of acenaphthylene (1). Remove the ice bath, react at room temperature for 25min, that is, pour ice-distilled water prepared in advance into it to terminate the reaction. Wash with saturated sodium carbonate solution and water, dry, and rotary evaporate. Diethyl ether / n-hexane = 1:3 (V:V) was used as the solvent to recrystallize the product to obtain light yellow solid powder (2) with a yield of 58% and a melting point of 67.0-68.5°C.
[0036] Synthesis of 5-(4-ene-valeryl)-acenaphthene (3) Put 30ml of benzene into a flask and heat up to 50°C, add 3.9g of potassium tert-butoxide powder and 4.2g of propylene bromide; take another 35ml...
Embodiment 2
[0044] This example is the preparation of 5-[1-(3,5-dinitrobenzoimide)-10-undecenoyl]acenaphthene compound and its chiral substance.
[0045] The synthesis of 5-acetyl acenaphthylene (2) is exactly the same as in Example 1, omitted.
[0046] Synthesis of 5-(10-undecenoyl)-acenaphthene (3) Put 30ml of benzene into a flask and heat up to 50°C, add 3.9g of potassium tert-butoxide powder and 4.2g of bromodecadecene; another 40ml of benzene Raise the temperature to 40°C, add 6.0g of the product (2) to dissolve, drop this solution into the flask with a sample injector, and stir while adding; heat the mixture to 95°C, reflux for 3h, and stop the reaction. After the reaction solution was cooled, it was washed with water, dried, and rotary evaporated. Toluene was used for column chromatography to remove raw materials and by-products to obtain reddish-brown crystals (3) with a yield of 24%. The melting point is 113.0-115.0°C.
[0047] Synthesis of 5-(1-amino-10-undecenoyl)-acenaphthe...
Embodiment 3
[0054] This example is the preparation of 5-[1-(3,5-dinitrobenzoimide)-4-pentyl]-acenaphthene compound and its chiral substance.
[0055] Synthesis of 5-acetyl acenaphthylene (2) Take 12.0 g of anhydrous aluminum trichloride and dissolve it in 120 ml of dichloromethane, add 4.4 g of acetyl chloride while stirring in an ice bath, and finally add 10.4 g of acenaphthylene (1), remove Ice bath, react at room temperature for 25min, that is, ice distilled water prepared in advance is poured into it to terminate the reaction. Wash with saturated sodium carbonate solution and water, dry, and rotary evaporate. Diethyl ether / n-hexane = 1:3 (V:V) was used as the solvent to recrystallize the product to obtain light yellow solid powder (2) with a yield of 58% and a melting point of 69.0-71.0°C.
[0056] Synthesis of 5-(4-ene-pentyl)-acenaphthene (3) Put 30ml of benzene into a flask and heat up to 50°C, add 3.9g of potassium tert-butoxide powder and 4.2g of bromopropane; take another 35ml ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
| separation factor | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com