Nanometer antiviral liposome medicine and its prepn
A nano-liposome and drug technology, which is applied in antiviral agents, liposome delivery, pharmaceutical formulations, etc., can solve the problems of adverse reactions and low oral bioavailability, so as to avoid relapse, improve blood circulation and Microcirculation, the effect of improving curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The present embodiment provides a kind of nano liposome medicine, comprises alprostadil and adefovir dipivoxil, comprises the raw material of following parts by weight:
[0030] Alprostadil and adefovir dipivoxil
[0031] Mixture 10.1 in a weight ratio of 0.1:50;
[0032] Soy lecithin 88;
[0033] Cholesterol and soybean sterol weight ratio 9: 1 mixture 40;
[0034] Equal weight mixture of polyethylene glycol 4000 and polyethylene glycol 2000 15;
[0035] Sodium Glucuronate 100;
[0036] Vitamin C 160;
[0037] Reduced Glutathione 5.
[0038] The preparation method of above-mentioned medicine, the steps are as follows:
[0039] (1) get the mixture of alprostadil and adefovir dipivoxil weight ratio 0.1-1.0: 50-300 of said amount, soybean lecithin, cholesterol and soybean sterol weight ratio 9: 1 mixture and dissolve in ethanol or ether, prepare into solution;
[0040] (2) Get the solution prepared in step (1) and evaporate to dryness in a rotary evaporator to mak...
Embodiment 2
[0052] The present embodiment provides a kind of nano liposome medicine, comprises alprostadil and adefovir dipivoxil, comprises the raw material of following parts by weight:
[0053] Alprostadil and adefovir dipivoxil
[0054]Mixture with a weight ratio of 0.65:260 56.7
[0055] Soy Lecithin 368;
[0056] Cholesterol and soybean sterol weight ratio 9:1 mixture 181;
[0057] Equal weight mixture of polyethylene glycol 4000 and polyethylene glycol 2000 31;
[0058] Sodium Glucuronate 240;
[0059] Vitamin C 723;
[0060] Reduced glutathione 7.8.
[0061] The preparation method of above-mentioned medicine refers to embodiment 1.
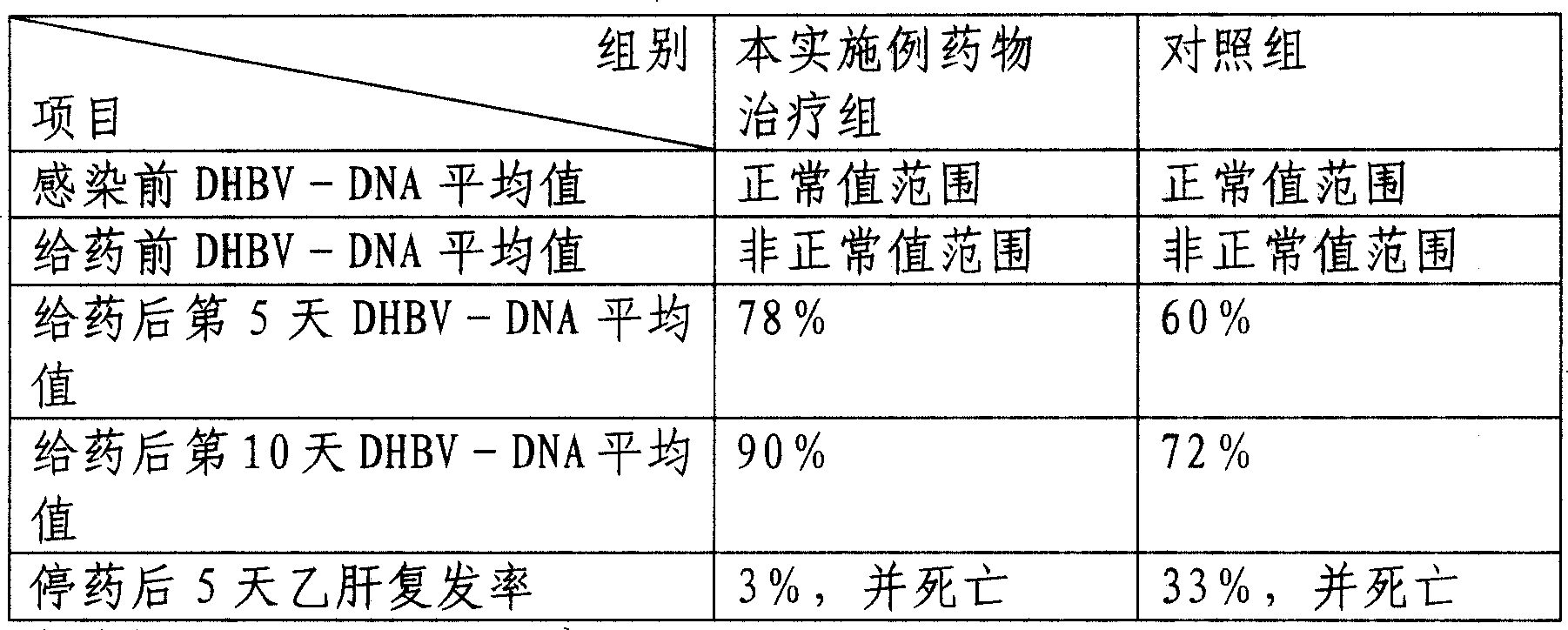
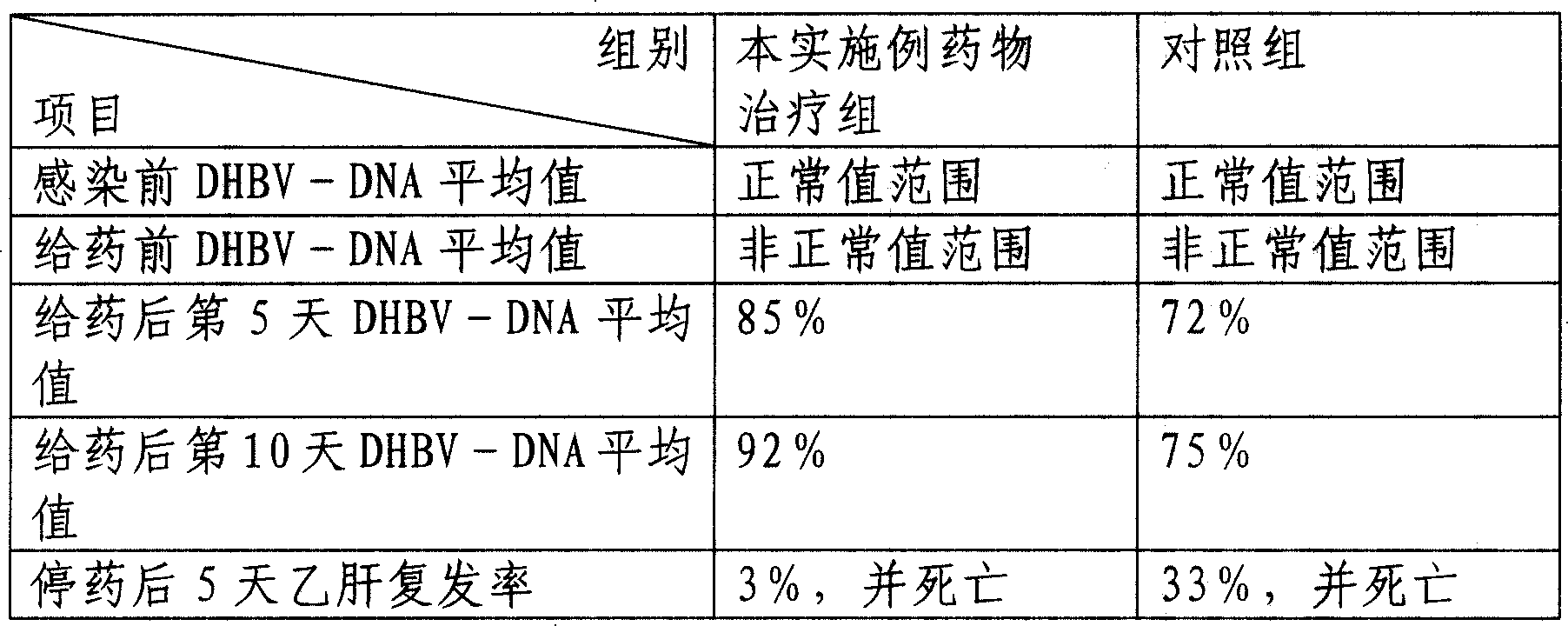
[0062] The curative effect verification of the medicine prepared in the present embodiment is as follows:
[0063] One-day-old Beijing female ducks were taken as a model infected with hepatitis B virus, and administered on the eighth day after infection. . Before infection, 7 days after infection, on the 5th and 10th day after administration,...
Embodiment 3
[0068] The present embodiment provides a kind of nano liposome medicine, comprises alprostadil and adefovir dipivoxil, comprises the raw material of following parts by weight:
[0069] Alprostadil and adefovir dipivoxil
[0070] Mixture 80.2 in a weight ratio of 1.0:300;
[0071] Soy Lecithin 440;
[0072] Cholesterol and soybean sterol weight ratio 9: 1 mixture 200;
[0073] Equal weight mixture of polyethylene glycol 4000 and polyethylene glycol 2000 40;
[0074] Sodium Glucuronate 280;
[0075] Vitamin C 800;
[0076] Reduced Glutathione 10.
[0077] The preparation method of above-mentioned medicine refers to embodiment 1.
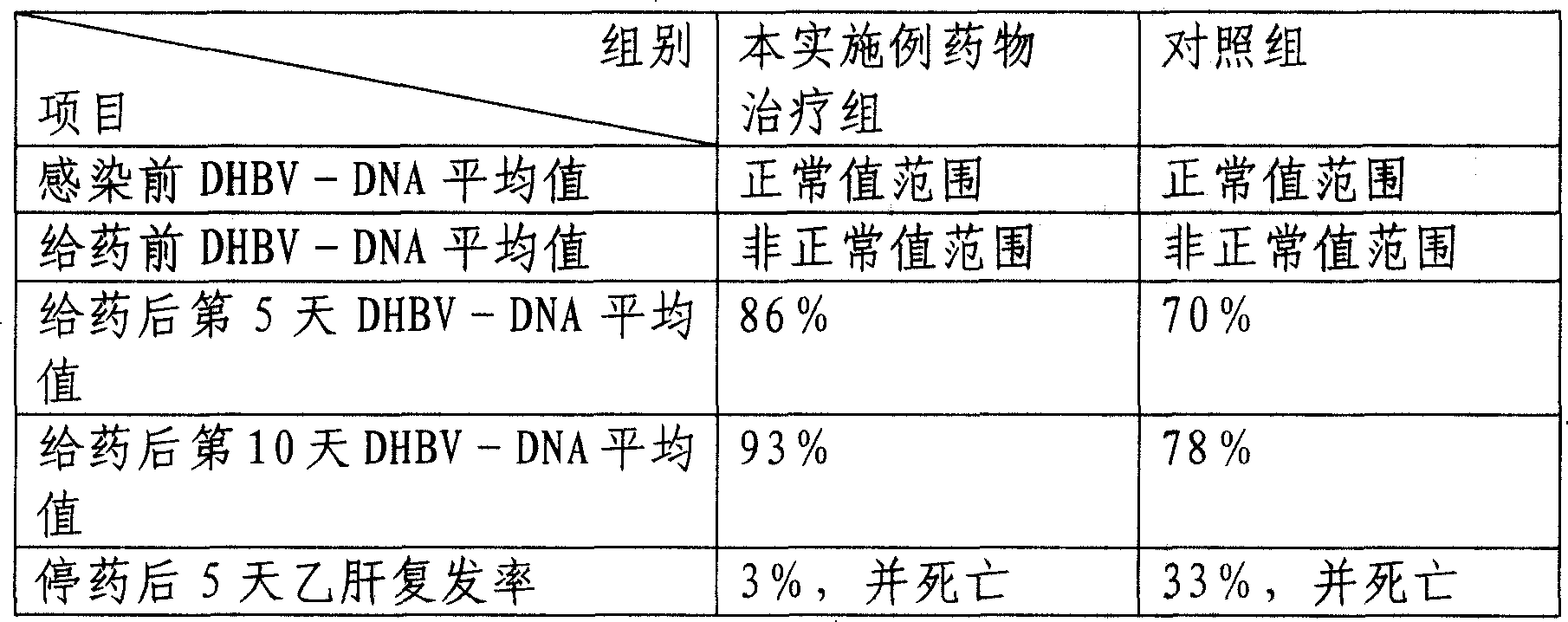
[0078] The curative effect verification of the medicine prepared in the present embodiment is as follows:
[0079] One-day-old Beijing female ducks were taken as a model infected with hepatitis B virus, and administered on the eighth day after infection. . Before infection, 7 days after infection, on the 5th and 10th day after administration, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com