Method for measuring drug fast variation of hepatitis b virus to Adefovir
A hepatitis B virus and drug-resistant technology, applied in the field of medical testing, can solve the problems of cumbersome operation, time-consuming, lack of detection methods, etc., and achieve the effect of improving detection efficiency, high sensitivity and accuracy, and intuitive and easy-to-judge detection results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
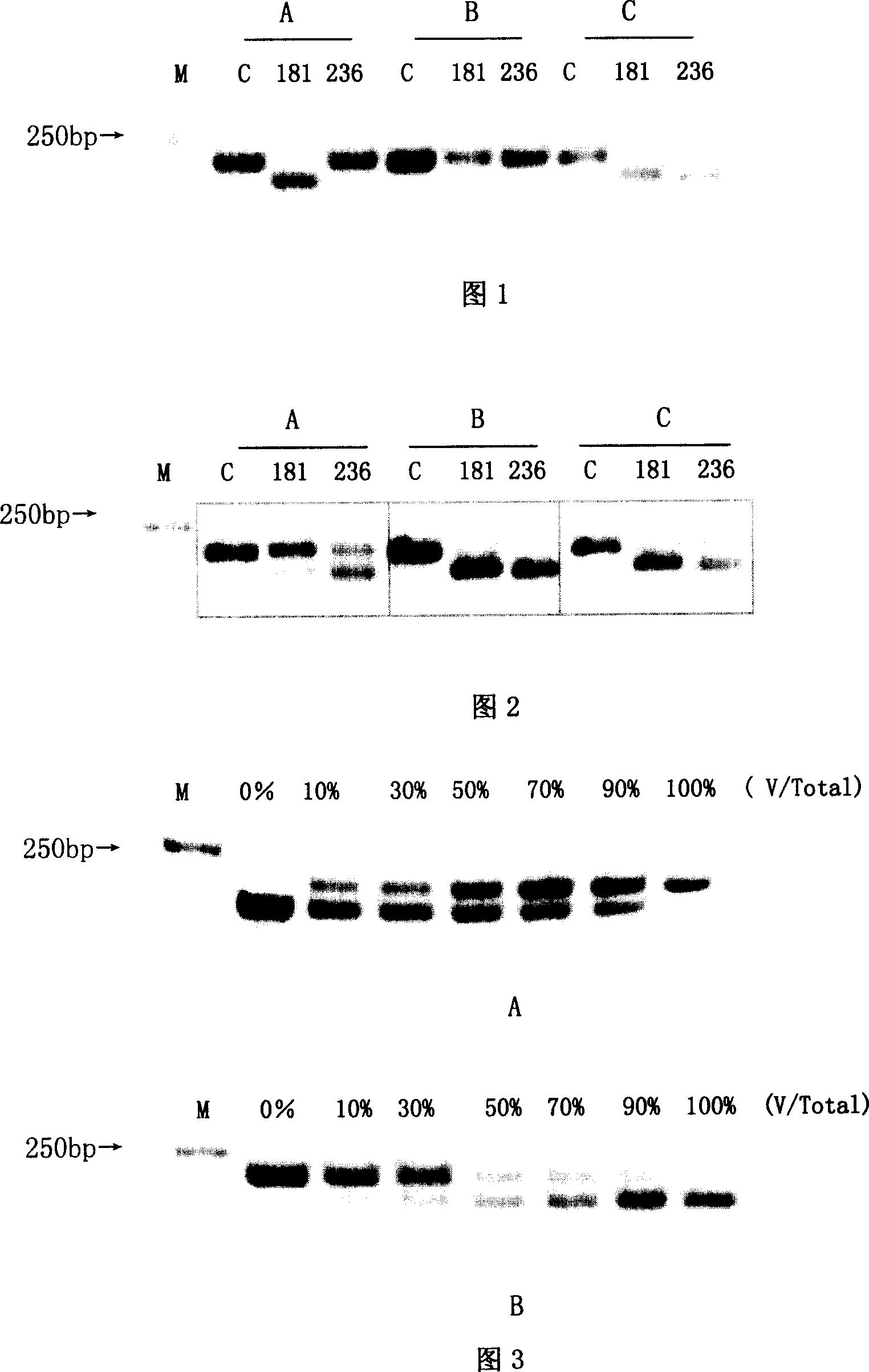
[0047] (1) Extract the HBV DNA in the patient's serum by classical alkaline lysis-phenol / chloroform extraction method, add 100 μl serum to 300 μl TES solution (10mM Tris-HCl, pH8.0, 5mM EDTA, 0.5% SDS, 50μg Protease K), mix Digest at 65°C for 3 hours, extract with equal volumes of phenol / chloroform and chloroform once each, add 2 times the volume of absolute ethanol and 1 / 10 volume of 3M sodium acetate (pH 4.8) to the supernatant to precipitate DNA, rinse once with 70% ethanol, The DNA was dissolved in 20 μl sterilized double distilled water and stored at -70°C for later use.
[0048] (2) Design of PCR-RFLP detection method ADV drug resistance-related mutations mainly involved the changes of 2 amino acids, namely 181 and 236. Before designing the PCR-RFLP detection method, it is necessary to understand the polymorphism analysis of the nucleotide sequence encoding the 181, 236 and adjacent amino acids of the RT region. For this reason, the present invention downloads the whole...
Embodiment 2
[0053] Sensitivity experiment to verify this method-cloning of RT region gene fragments containing ADV drug resistance-related mutations
[0054] PCR primers (Table 3) were designed on both sides of the A-E motif in the RT region of HBV polymerase, and the HBV DNA extracted above was used as a template to amplify by nested PCR. The upstream and downstream primers of the first round were RTS1 and RTAS1 respectively, and the upstream and downstream primers of the second round of PCR were RTS2 and RTAS2. The PCR amplification conditions were: 94°C for 30s, 56°C for 60s, and 72°C for 45s, a total of 35 cycles. The system is 50ul. PCR amplification uses Premix Taq enzyme. The PCR products were directly sequenced after gel extraction and purification. The PMD18-T Vector TA cloning kit was used to screen the clone containing the target fragment, and the plasmid was identified by SacI and PstI double enzyme digestion and sequencing analysis. 10 cloned plasmids were sequenced for ea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com