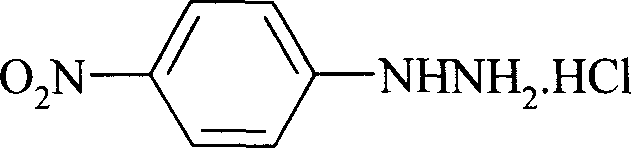
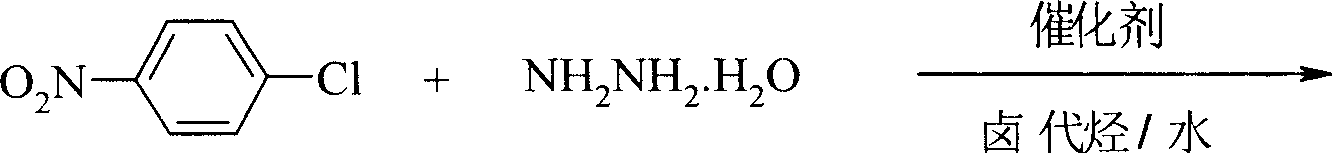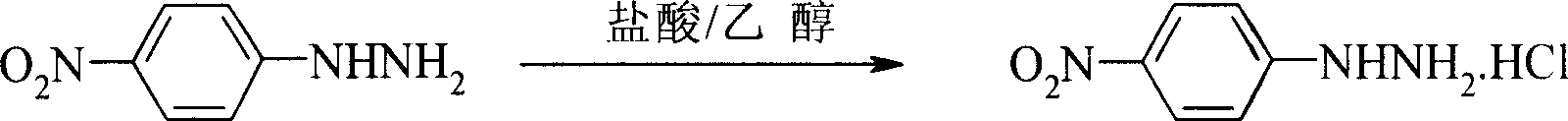Preparation process of p-nitro phenyl hydrazine hydrochloride
A technology of nitrophenylhydrazine and p-chloronitrobenzene, which is applied in the preparation of hydrazine, organic chemistry, etc., can solve the problems of long reaction steps, low product yield, and high raw material price, and achieve low cost, good product purity, and high yield. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Add 150ml chloroform, 78.8g (0.5mol) p-chloronitrobenzene respectively in the 500ml reaction bottle that has stirrer, thermometer, reflux condenser, stir and dissolve at room temperature, add 100ml water under stirring, 34.4g (0.55mol) 80 % hydrazine hydrate, 1.2 g 18-crown-6 and 1.2 g sodium fluoride were added. Heat up, react at 50-55°C for 4.5 hours, cool the reaction solution to room temperature, let stand to separate the organic phase, evaporate chloroform under reduced pressure, add 45ml of 37% hydrochloric acid and 150ml of 95% ethanol to the obtained p-nitrophenylhydrazine, and stir While gradually heating up to 70°C, and cooling down to 0-5°C after complete dissolution, the filtered solid was dried to obtain 78.3g of p-nitrophenylhydrazine hydrochloride orange-red needle crystals, with a yield of 82.6% and a purity of 99.3%. (HPLC), melting point 204-205°C.
Embodiment 2
[0023] Add 160ml of 1,2-dichloroethane and 78.8g (0.5mol) p-chloronitrobenzene respectively in a 500ml reaction flask with a stirrer, a thermometer, and a reflux condenser, stir and dissolve at room temperature, add 120ml of water under stirring, 36.3 g (0.58mol) 80% hydrazine hydrate, add 1.4g 15-crown-5 and 1.4g potassium fluoride. Heating and raising the temperature, reacting at 55-60°C for 5 hours, cooling the reaction liquid to room temperature, standing to separate the organic phase, distilling off 1,2-dichloroethane under reduced pressure, adding 45ml of 37% hydrochloric acid to the obtained p-nitrophenylhydrazine, In 150ml of 95% ethanol, gradually heat up to 70°C while stirring, cool down to 5-10°C after completely dissolving, and dry the filtered solid to obtain 80.4g of p-nitrophenylhydrazine hydrochloride orange-red needle crystals. The yield is 84.8%, the purity is 99.4% (HPLC), and the melting point is 204.5-205°C.
[0024] With the same method in the foregoing ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com