No-cell calmette-Guerin bacillus mucosa immune regulator and application thereof
A technique for mucosal immunity and BCG, applied in the biological field, can solve problems such as unsatisfactory drug efficacy, and achieve the effects of preventing the recurrence of condyloma acuminatum, promoting recovery, and preventing precancerous lesions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Example 1: Preparation of acellular BCG mucosal immune modulator
[0088] Different physical lysis methods can be used to prepare the acellular BCG mucosal immune modulator, such as ultrasonic crushing or high-pressure crushing, and high-pressure crushing is most preferred.
[0089] The BCG strain used for production is D 2 PB 302 S 11 First 10 Strain (preserved by Shanghai Institute of Biological Products), culture characteristics, toxicity test, safety test, spleen activation test, tumor inhibition test, toxicity test, etc. were carried out on it before production. The strains that pass the above six inspections can be used for production. It is stipulated in the production that the generation of the strain cannot exceed 12 generations.
[0090] It is strictly forbidden to use the strains of animals to manufacture BCG.
[0091] Preparation of cell-free BCG mucosal immune modulator by high pressure crushing method
[0092] 1.1 Strain subculture and cultivation ...
Embodiment 2
[0112] Example 2: Effect of acellular BCG mucosal immune modulator on immune function of tumor-bearing mice
[0113] This example mainly observes the effect of acellular BCG mucosal immune conditioning agent on the content of serum lysozyme in tumor-bearing mice and delayed hypersensitivity (PTH) by intragastric administration to illustrate the effect of acellular BCG mucosal immune conditioning agent on Effects on the immune function of tumor-bearing mice.
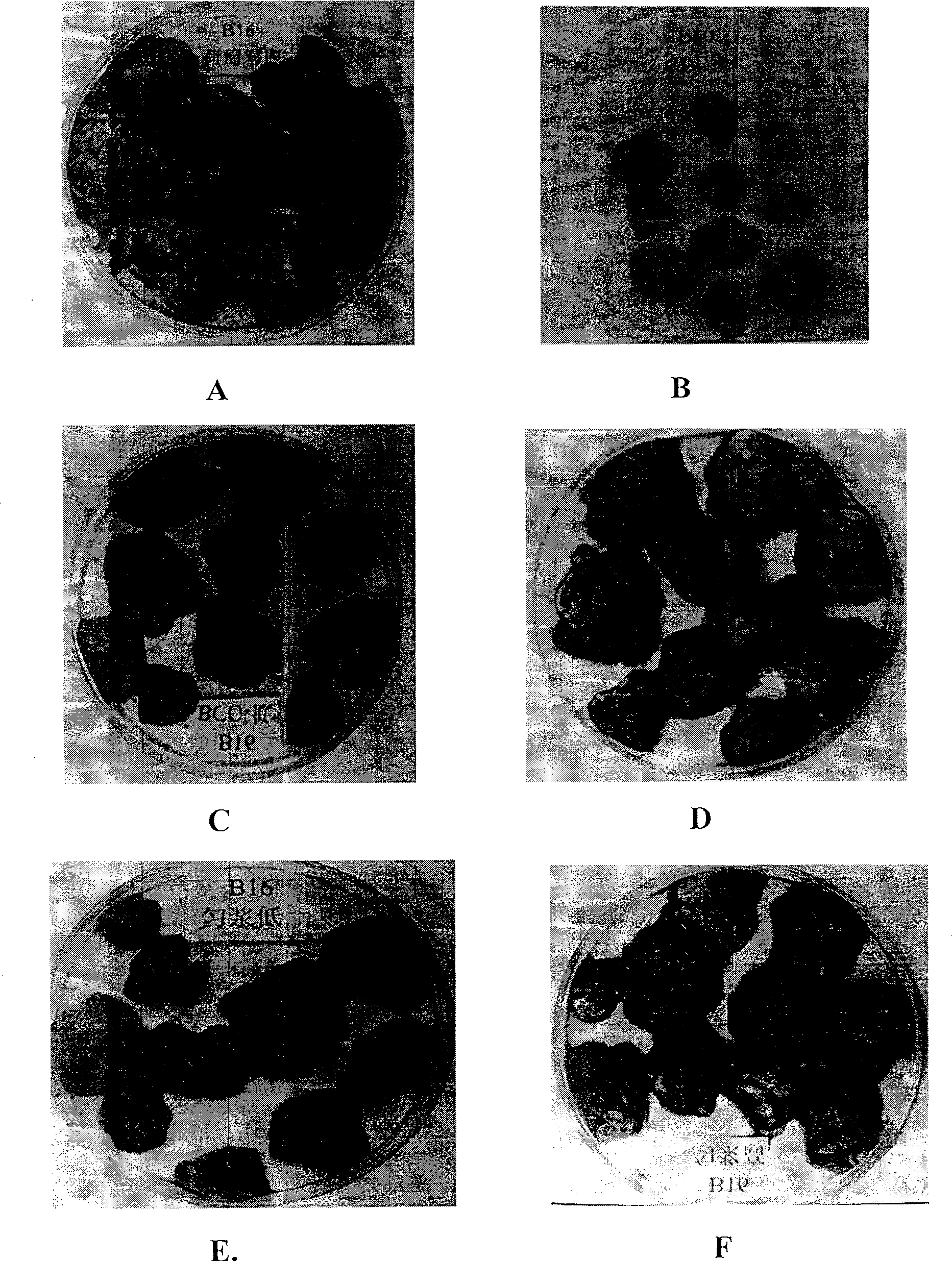
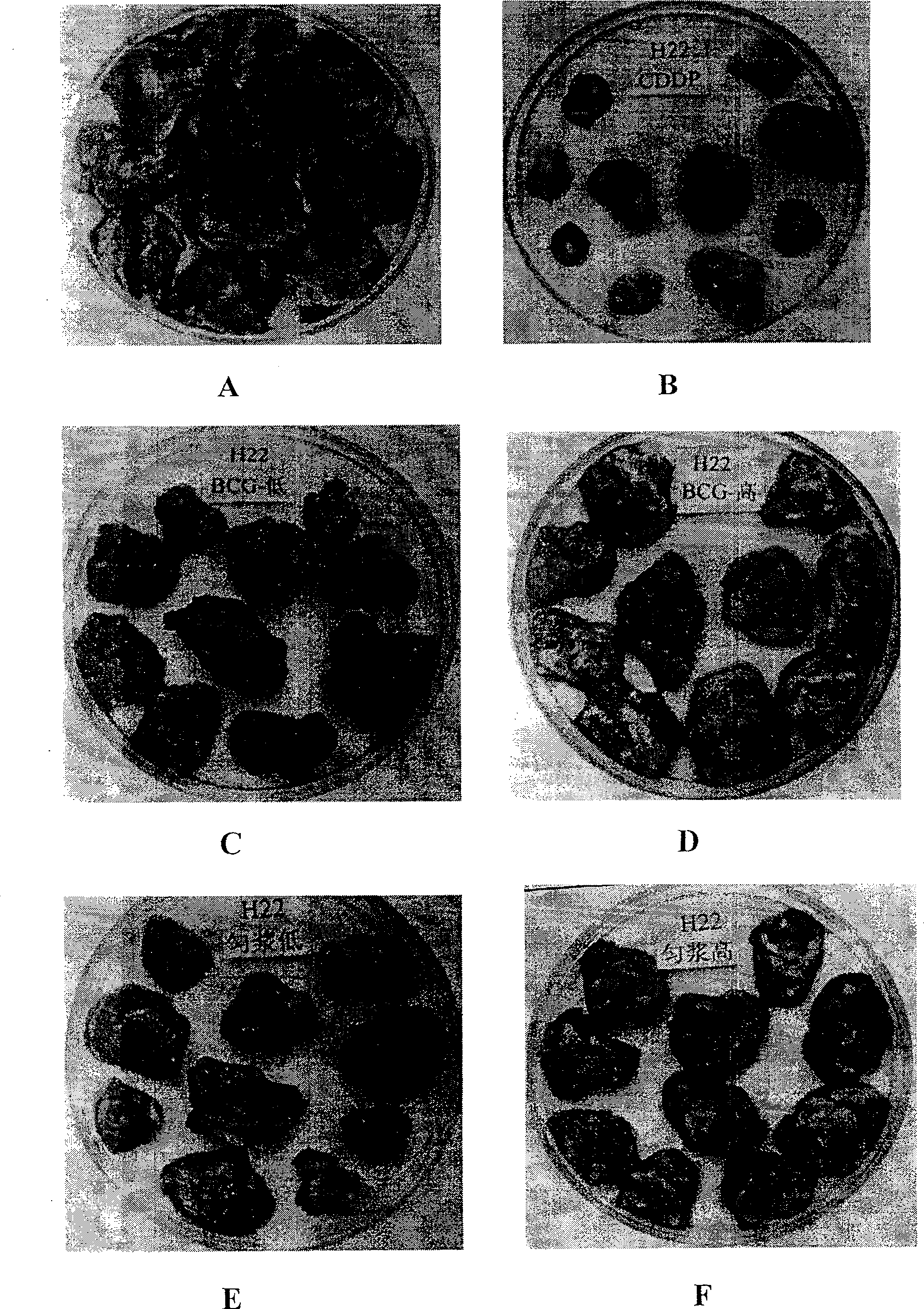
[0114] 2.1 Effect of cell-free BCG mucosal immunomodulator on serum lysozyme content of melanoma B16 tumor-bearing mice
[0115] According to the concentration of melanoma B16 cells at 1:3, 0.2ml was inoculated subcutaneously or intraperitoneally to mice, and then administered by intragastric administration 24 hours later. There are three dosage groups for acellular BCG mucosal immune conditioning agent, 25.00U / kg is selected as the high dosage group, 12.50U / kg is selected as the middle dosage group, and 6.25U / kg is sele...
Embodiment 3
[0127] Example 3: Effect of acellular BCG mucosal immune modulator on immune function of immunocompromised body
[0128] This example illustrates the effect of acellular BCG mucosal immune modulators on the immune function of immunocompromised mice induced by cyclophosphamide.
[0129] Seventy Balb / c mice were randomly divided into 5 groups, namely low, medium and high doses of acellular BCG mucosal immune modulator, negative control group and normal control group, 14 in each group, half male and half male.
[0130] The control group with normal immune function was not given any drugs;
[0131] The remaining experimental groups:
[0132] a. The positive control group was fed with 0.2ml of A collagen solution every other day for a total of 10 times.
[0133] b. The negative control group was fed with 0.2ml of normal saline every other day for a total of 10 times.
[0134] c. The high-dose group was given 20U / 0.2ml of acellular BCG mucosal immune conditioning agent every othe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com