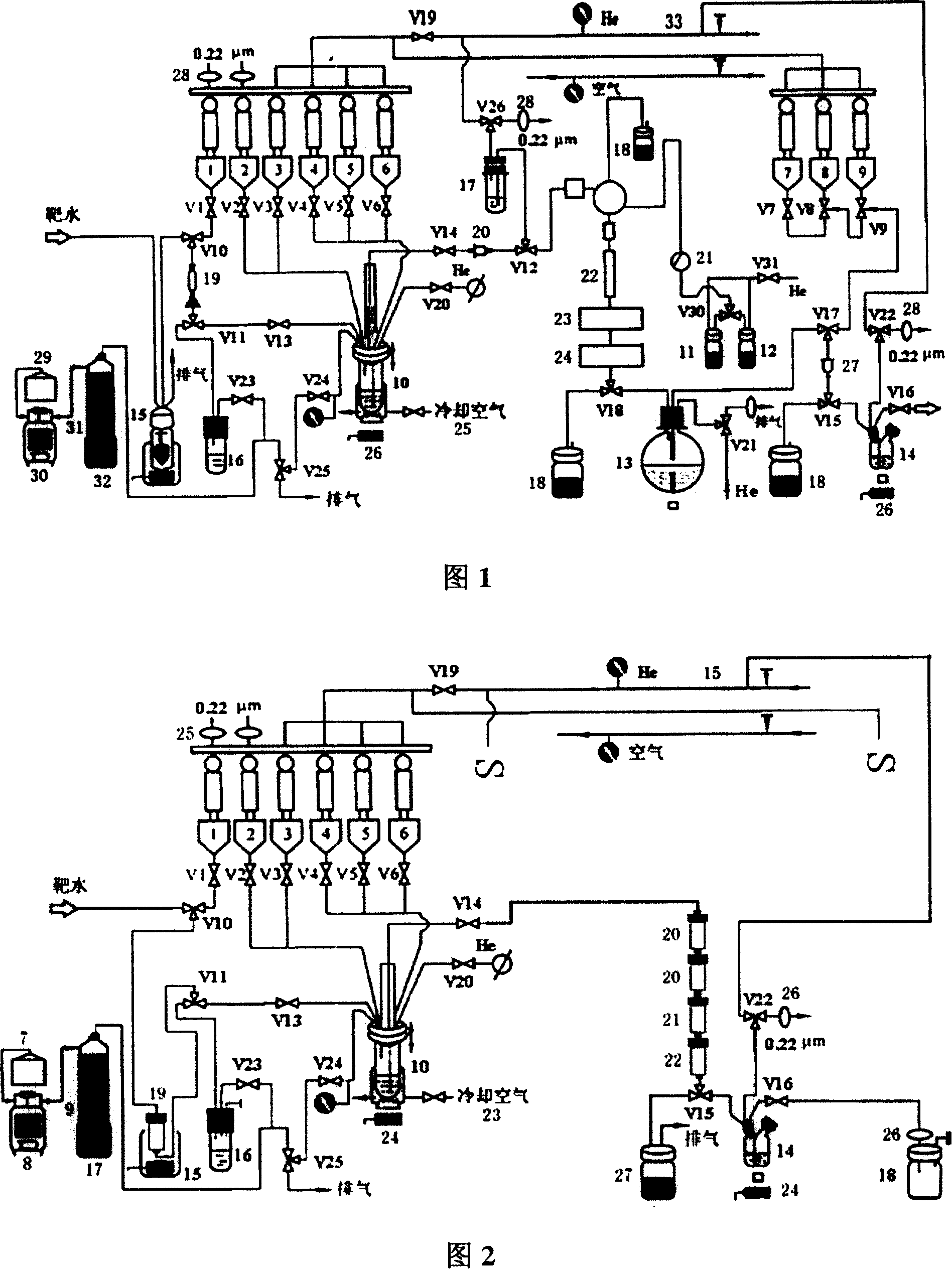
2-18F-2-deoxidized-D-glucose synthesis process
A synthesis process and glucose technology are applied in the synthesis process field of radiopharmaceutical 2-18F-2-deoxy-D-glucose, and can solve the problems such as inability to completely remove toxic substance K222, high production cost, increase in total synthesis time, and the like, To achieve the effect of controllable synthesis process, shorten synthesis time, and reduce synthesis time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0041] Embodiment: the synthesis technique described in the present invention is applied to existing TRACERlab FX F-N Automated Synthesis System
[0042] 1 Materials and methods
[0043] 1.1 Instruments and reagents
[0044] PET trace Cyclotron and Tracerlab FX F-N Automated synthesis system, product of GE Company of the United States (http: / / www.gehealthcare.com); LC-10AT HPLC analysis system, product of Shimadzu Company of Japan, equipped with LB 508 radioactive flow detector, product of EG&G Company of Germany; CS-9301PC Thin-layer tomography scanner, product of Shimadzu Company, Japan; gamma counter, product of Shanghai Institute of Nuclear Research; CRC-15R activity meter, product of CAPINTEC Company of the United States. Acetonitrile, product of Aldrich Company; 4,7,13,16,21,24-hexaoxa-1,10-diazabicyclo[8,8,8]hexacane (Kryptofix2.2.2, K222), Germany Merck company product; 1,3,4,6-tetra-O-acetyl-2-O-trifluoromethanesulfonyl-β-D-mannose (referred to as trifluoromannose...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com