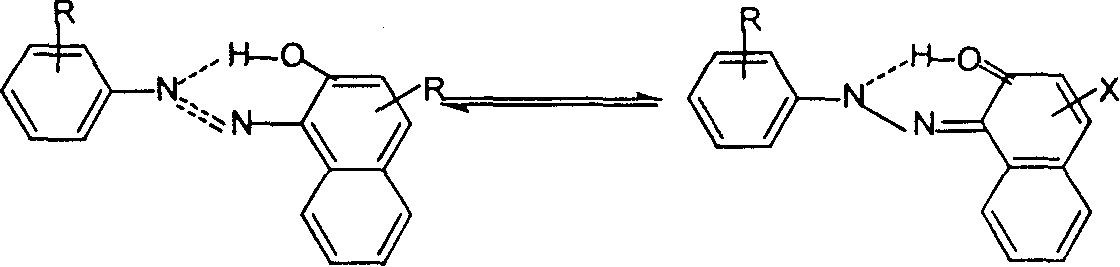
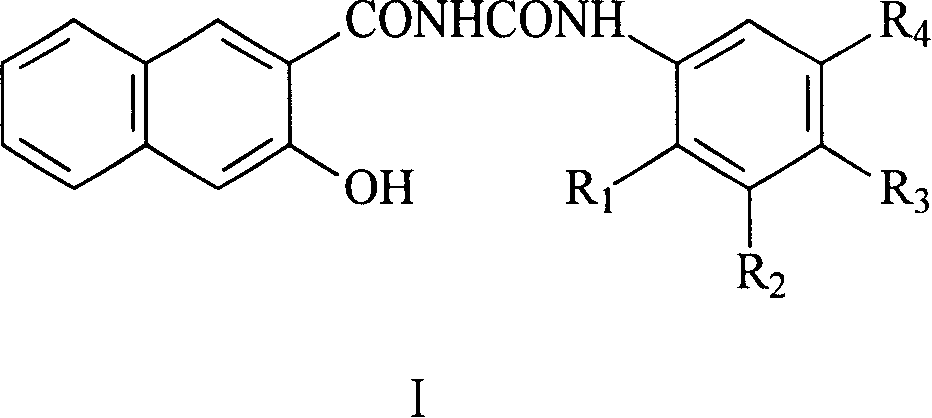
Naphthurea composition for preparation of carrier generation materials and process for preparing same
A naphthoyl urea and compound technology, which is applied in the field of intermediates of carrier generation materials, to achieve the effects of low raw material prices, good photoelectric properties, and simple synthesis routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0018] Example 1: Synthesis of N-(3,5-dimethylphenyl)-N'-(3-hydroxyl-2-naphthoyl)urea
[0019] [1] Synthesis of 3-hydroxy-2-naphthoyl chloride
[0020] In a 250mL four-necked flask equipped with a reflux condenser, a thermometer and a stirrer, add 90.0mL carbon tetrachloride, 44.0mL (0.60mol) thionyl chloride and 0.5mL DMF, and finally add 2-hydroxy-3-naphthalene 37.6g (0.20mol) of formic acid was fully stirred, and the reaction temperature was controlled at 50°C. Stop the reaction until the yellow suspension turns into a red clear liquid, and place it at 0-10°C for 2-3 hours to precipitate the product. Then, it was filtered and dried to obtain 36.2 g of 2-hydroxy-3-naphthoyl chloride with a yield of 87.5%.
[0021] [2] Synthesis of 3,5-dimethylphenylurea
[0022] First prepare sodium cyanate solution, dissolve 19.50g (0.30mol) sodium cyanate with 90.0mL water, if it is still not completely dissolved, heat it a little. Then in a 500mL beaker, add 40.0mL of glacial acetic a...
example 2
[0025] Example 2: Synthesis of N-(4-methylphenyl)-N'-(3-hydroxyl-2-naphthoyl)urea
[0026] [1] Same as Step 1 in Example 1
[0027] [2] Synthesis of p-methylphenylurea
[0028] First prepare sodium cyanate solution, dissolve 19.50g (0.30mol) sodium cyanate with 90.0mL water, if it is still not completely dissolved, heat it a little. Then in a 500mL beaker, add 20.0mL of glacial acetic acid and 70.0mL of water, and add 10.72g (0.10mol) of p-methylaniline at 25°C. Then slowly add sodium cyanate solution dropwise to the beaker, and keep stirring until a small amount of precipitation appears in the solution, then quickly add the remaining sodium cyanate solution under rapid stirring, a large amount of white foam will be generated and the temperature will start to rise to 50-60°C . After stirring for 20 minutes, the reaction was stopped. Cool to room temperature, filter, wash with water, and dry to obtain 13.91 g of p-methylphenylurea with a yield of 92.6%.
[0029] [3] Synthe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com