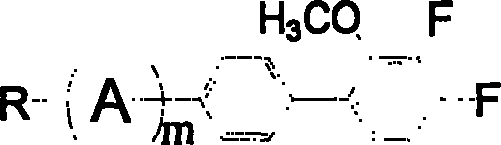3,4-difluoro-2-methoxyl biphenyl derivative and its preparation process and application
A technology of methoxybiphenyl and methoxybromobenzene, which can be used in ether preparation, chemical instruments and methods, liquid crystal materials, etc., can solve the problems of low viscosity, poor solubility, and high melting point, and achieves lower melting point and wider Application prospects, the effect of increasing solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064]Embodiment 1, preparation 4'-ethyl-3,4-difluoro-2-methoxybiphenyl
[0065] 1. Preparation of 3,4-difluoro-2-methoxyphenylboronic acid
[0066] Add 22.3g (0.1mol) of 3,4-difluoro-2-methoxybromobenzene, 50ml of tetrahydrofuran, 2.43g (0.1mol) of magnesium metal and 1 to 2 grains of iodine into a 250ml three-necked flask, and initiate the reaction at room temperature Stir and react for 1 hour, then lower the temperature of the system to -70°C with ethanol-liquid nitrogen system, slowly add 10.4g (0.1mol) trimethyl borate dropwise at this temperature, and make it under stirring condition after the dropwise addition is completed. Naturally rise to room temperature, then dropwise add 10% HCl solution to pH 1, heat to 100°C and reflux for 1 hour. After the reaction solution was cooled, the aqueous phase was extracted with 100ml×3 petroleum ether, washed with saturated brine until neutral, and dried overnight with anhydrous sodium sulfate. The dried solution was rotary evapora...
Embodiment 2
[0082] Example 2, preparation of 4'-(trans-4"-pentylcyclohexyl)-3,4-difluoro-2-methoxybiphenyl
[0083] 1. Preparation of 4'-(trans-4"-pentylcyclohexyl)phenylboronic acid
[0084] Add 30.9g (0.1mol) 4'-(trans-4"-pentylcyclohexyl) bromobenzene, 50ml tetrahydrofuran, 2.43g (0.1mol) metal magnesium and 1~2 grains of iodine to a 250ml three-necked flask, After initiating the reaction at low temperature, stir for 1 hour, then use the ethanol-liquid nitrogen system to lower the system temperature to -70°C, slowly add 10.4g (0.1mol) trimethyl borate dropwise at this temperature, and after the dropwise addition, under stirring conditions Allow it to rise to room temperature naturally, then add 10% HCl solution dropwise to pH 1, and heat to reflux at 100°C for 1 hour. After cooling the reaction solution, extract the aqueous phase with 100ml×3 petroleum ether, and then use saturated Wash with brine until neutral, and dry overnight with anhydrous sodium sulfate. The dried solution was e...
Embodiment 3
[0100] Example 3, preparation of 4"-pentyl-3,4-difluoro-2-methoxy-(1,1',4',1") terphenyl
[0101] Add 18.8g (0.1mol) 3,4-difluoro-2-methoxyphenylboronic acid obtained in Example 1 to a 500ml three-necked flask, 30.2g (0.1mol) 4'-pentyl-4-bromobiphenyl, 150ml Benzene, 75ml of absolute ethanol, 30g of sodium carbonate, 10g of water and 2.5g of tetrakis(triphenylphosphine)palladium were heated to reflux at 100°C for 24 hours and then cooled to room temperature. Separate the organic phase, extract the aqueous phase with 80ml×3 chloroform, combine the organic phases, wash with water until neutral, and dry with anhydrous sodium sulfate. The dried solution was rotary evaporated to remove the solution to obtain 27.5g of 4"-propyl-3,4-difluoro-2-methoxy-(1,1',4',1") terphenyl, yield 75% .
[0102] DSC (differential scanning calorimeter): Cr61.3I;
[0103] IR: Wavenumber / cm -1 =2954, 2931, 2856, 1915, 1884, 1610, 1491, 1468, 1427, 1408, 1392, 1315, 1275, 1230, 1176, 1092, 1049, 1005...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com