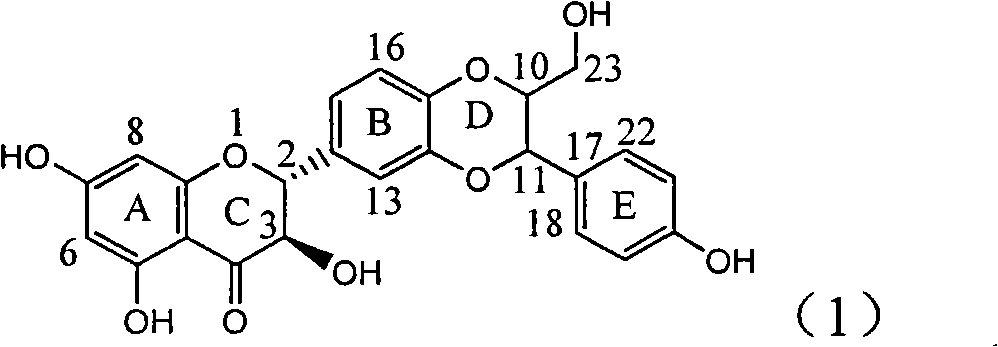
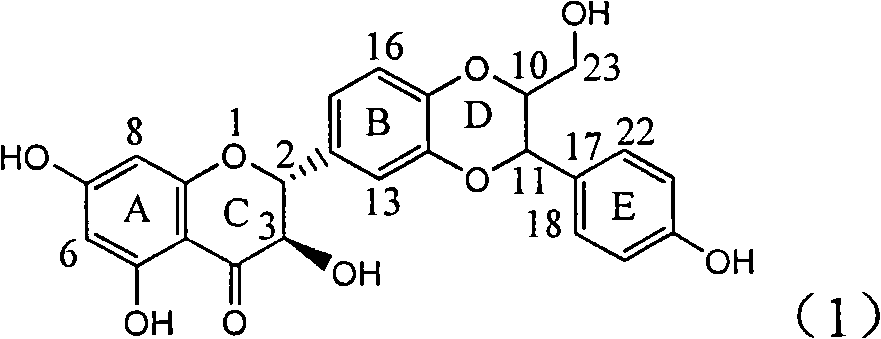
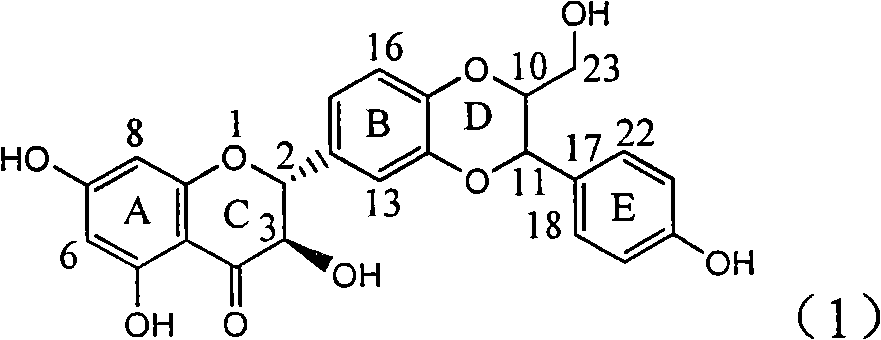
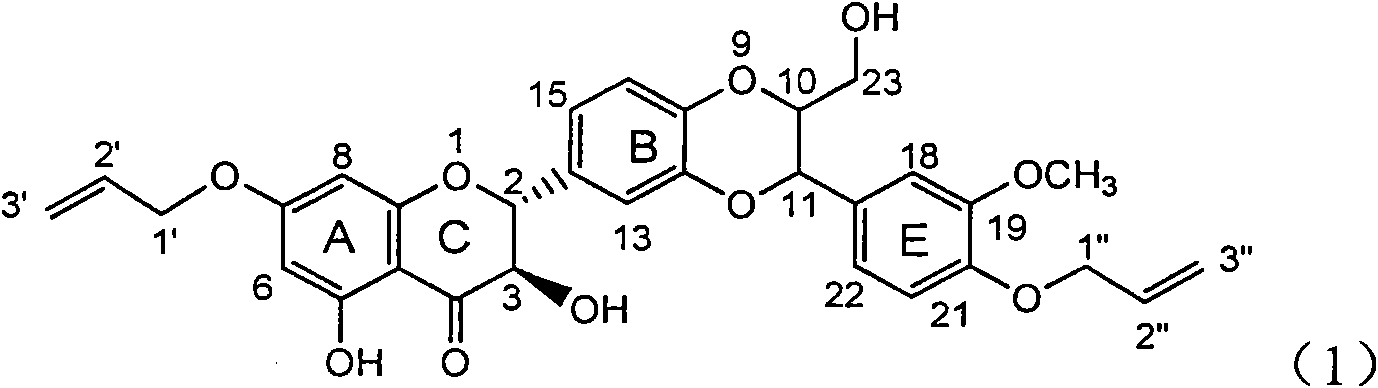
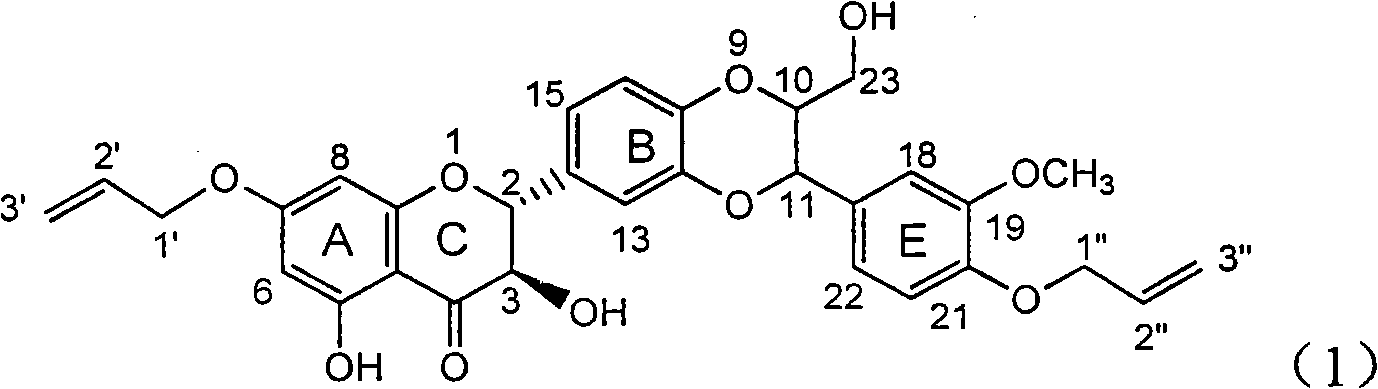
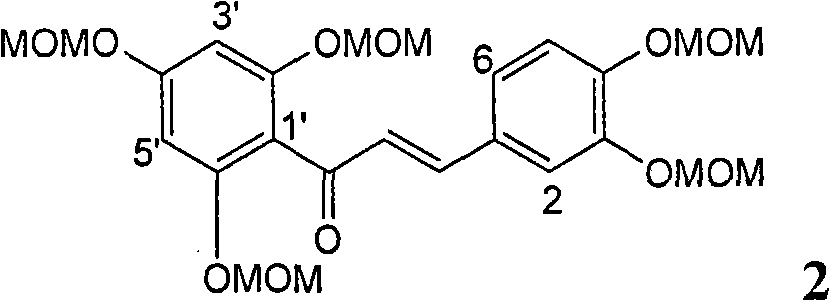
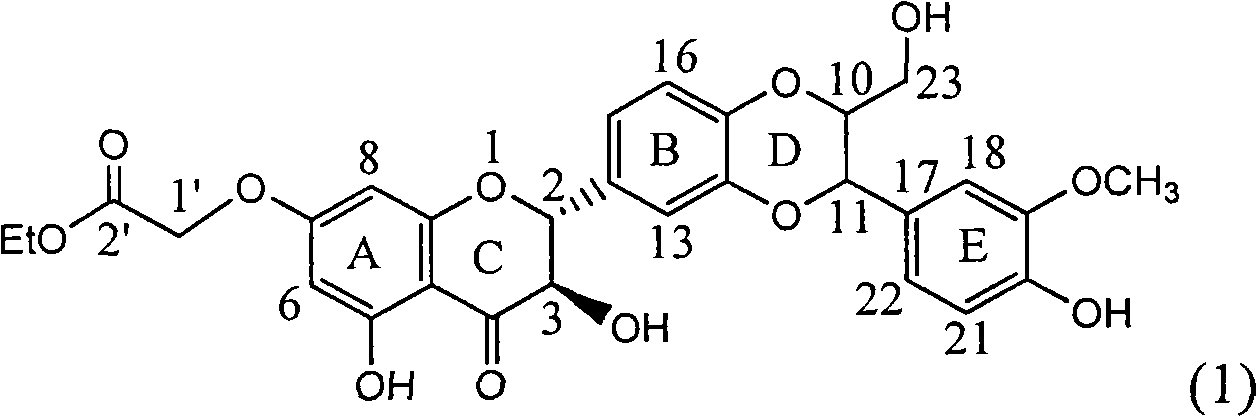
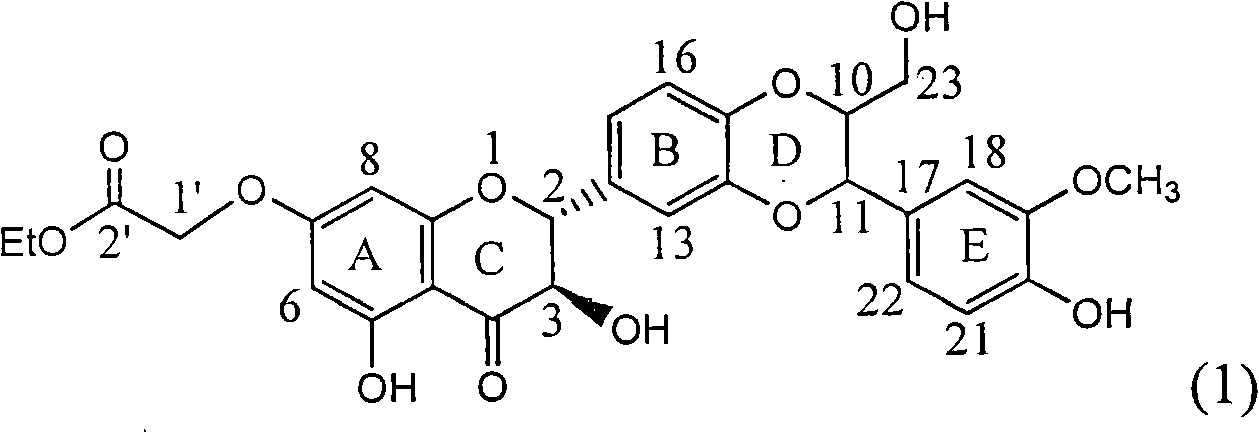
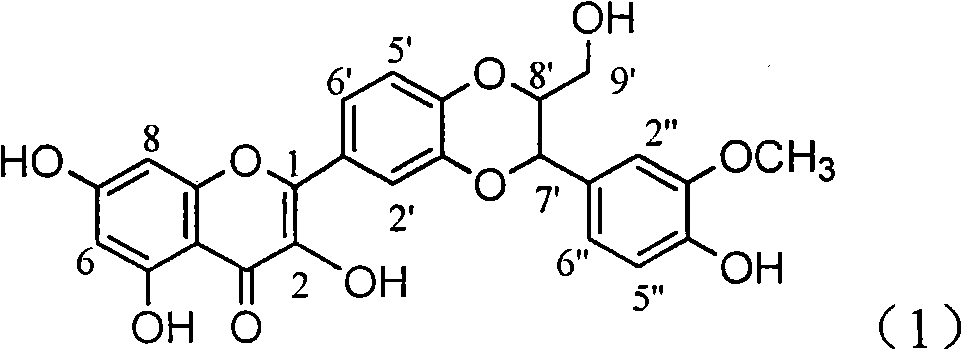
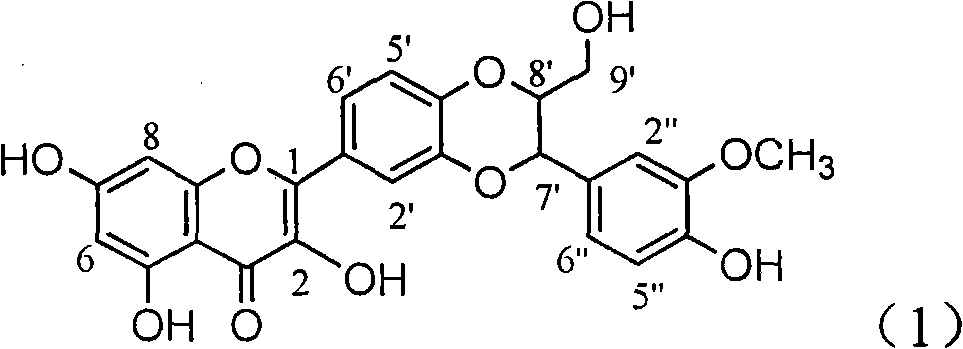
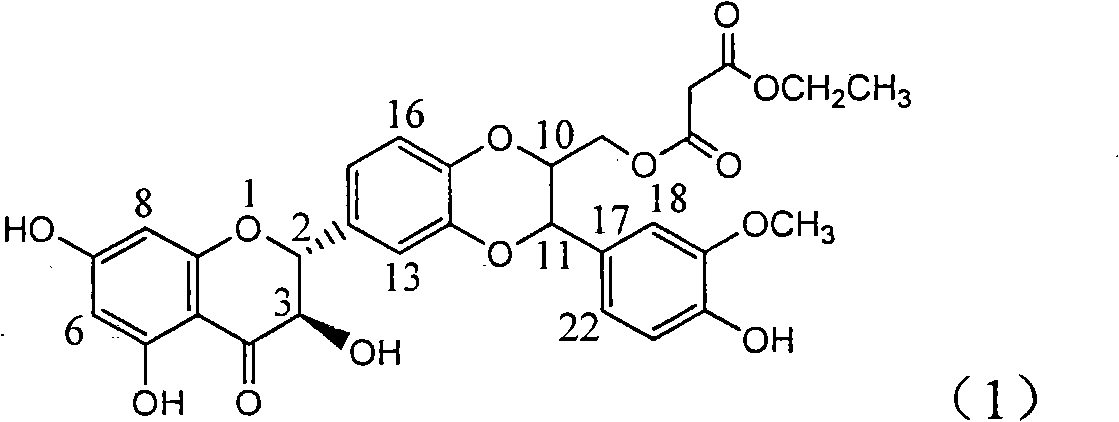
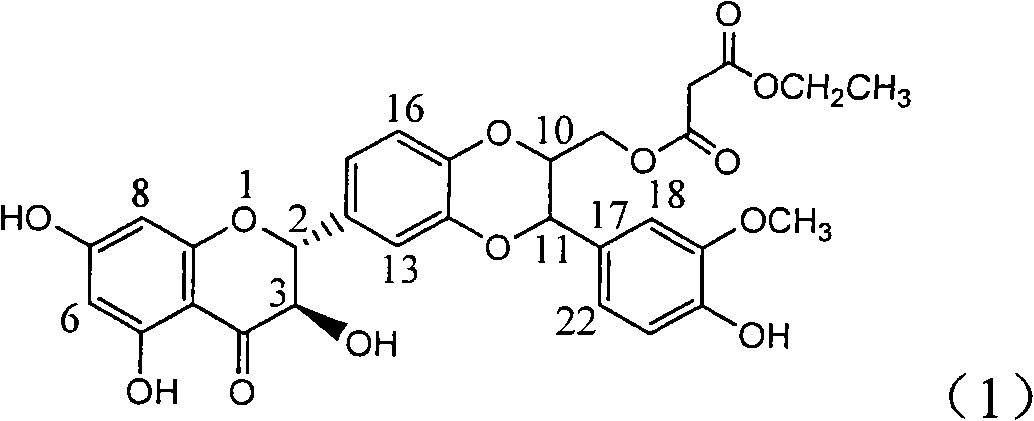
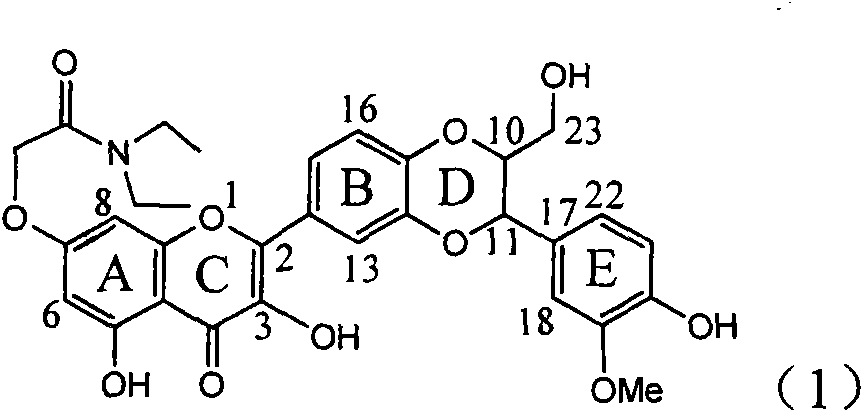
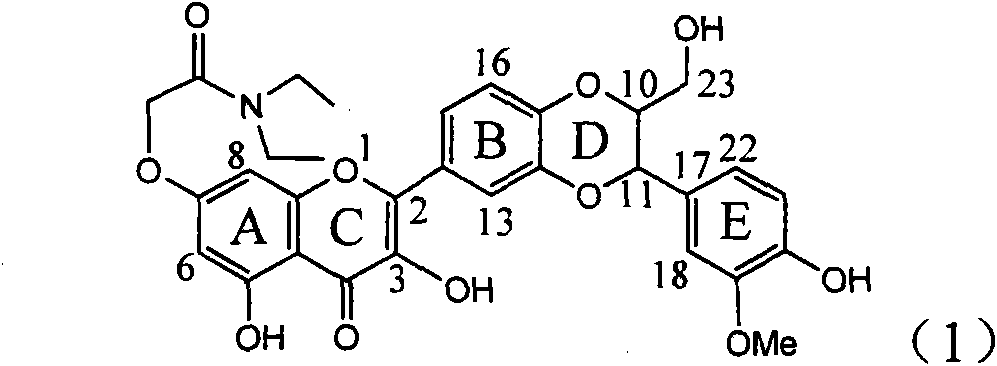
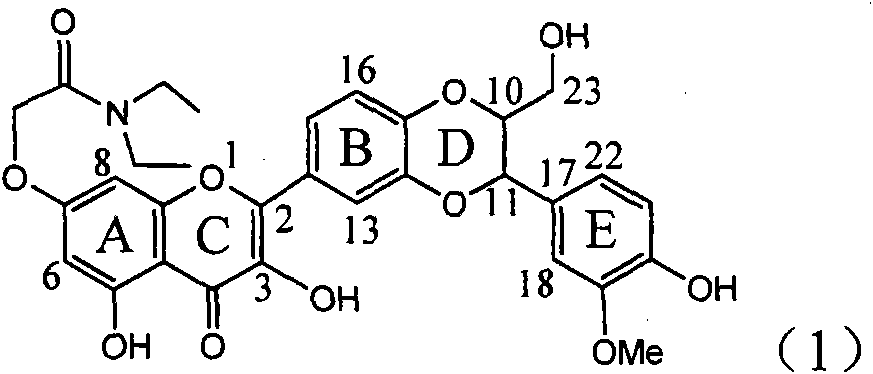
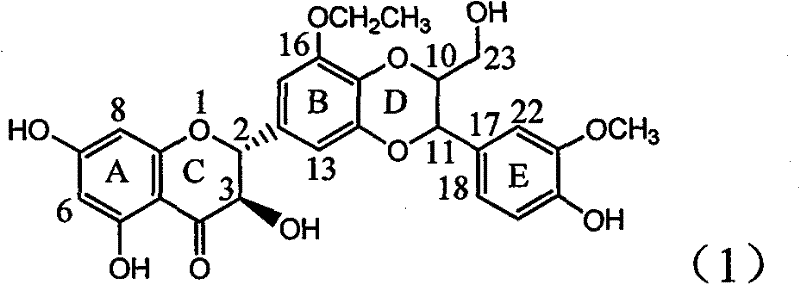
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
39results about How to "Convenient source of synthetic starting materials" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
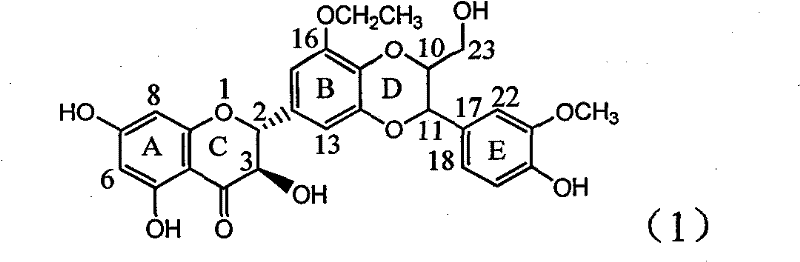
Use of acetamide dehydrogenation silibinin as medicament for treating viral hepatitis B
InactiveCN101829091APowerful removalInhibitory activityOrganic active ingredientsDigestive systemAntigenDisease
The invention relates to the use of acetamide dehydrogenation silibinin as a medicament for treating viral hepatitis B, in particular to the use of dehydrogenation silibinin esters flavonoid lignanoid replaced by A ring methoxy formyl amine or pharmaceutically acceptable salt as the medicament for eliminating HBsAg (hepatitis B surface antigen) and HBeAg (hepatitis Be antigen) and restraining copy of HBV DNA. The cetamide dehydrogenation silibinin can obviously restrain the HBsAg and HBeAg activity, and the strengths for eliminating the HBsAg and HBeAg are 90.5% and 63.6% at the concentration of 20 microgramme / milliter and are 5.6 times and 3.8 times more than positive contrast medicament alpha-interferon. Meanwhile, the restraining rate to the HBV DNA is 90.4% at the concentration, is 12% higher than lamivudine, and is 2.4 times more than a- interferon. Therefore, the flavonoid lignanoid or the pharmaceutically acceptable salt can be expected for treating hepatitis B virus infection as the non-nucleoside medicament.
Owner:DALI UNIV
Application of flavonoid quercetin dimmer as medicament for treating viral hepatitis B
InactiveCN101829103AConvenient sourceThe source is easy to getOrganic active ingredientsDigestive systemDiseasePositive control
The invention relates to the application of flavonoid quercetin dimmer as the medicament for treating viral hepatitis B, in particular to the application of flavonoid quercetin dimmer or pharmaceutically acceptable salt thereof to the preparation of the medicament for eliminating HBsAg and HBeAg and inhibiting HBV DNA replication. The flavonoid quercetin dimmer or pharmaceutically acceptable salt thereof has obvious HBsAg and HBeAg inhibiting activity, and at the concentration of 100mcg / ml, the flavonoid quercetin dimmer pharmaceutically acceptable salt thereof has the HBsAg eliminating strength of 65.7% and the HBeAg eliminating strength of 44.8% which are respectively 4.1 times and 2.7 times higher than the positive control medicament of Alpha-interferon and has the HBV DNA inhibiting ratio of 44.8% which is 117% of the HBV DNA inhibiting ratio of the Alpha-interferon at the highest test concentration. Therefore, the flavonoid quercetin dimmer or pharmaceutically acceptable salt thereof can be expectedly used for preparing the non-nucleoside medicament for eliminating HBsAg and HBeAg, inhibiting HBV DNA replication and treating viral hepatitis B.
Owner:DALI UNIV
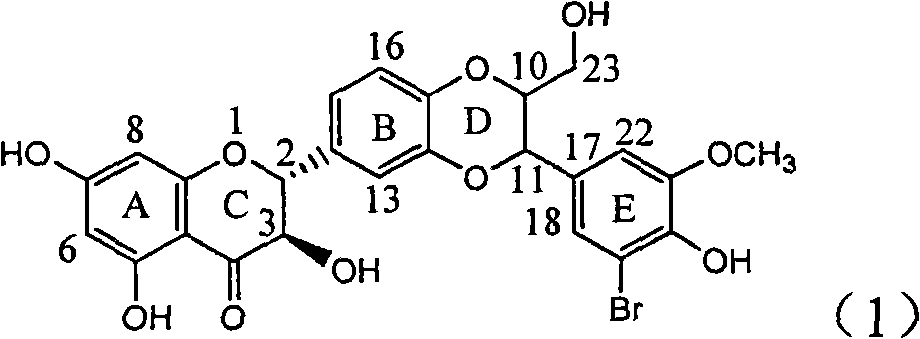
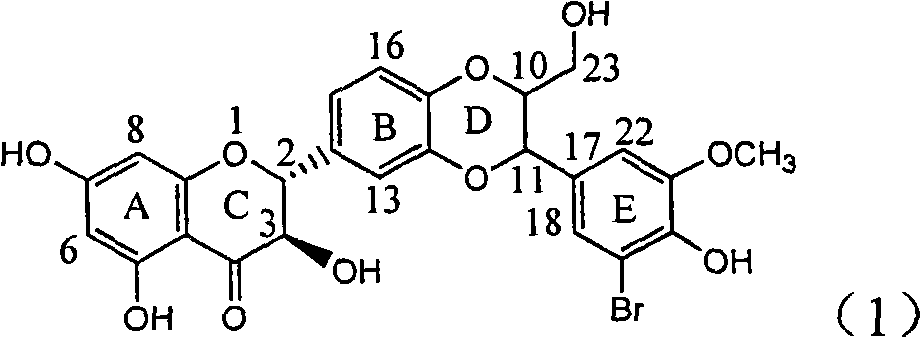
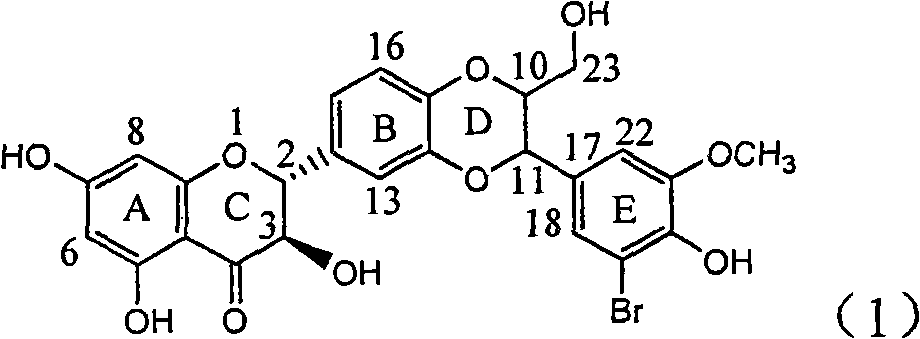
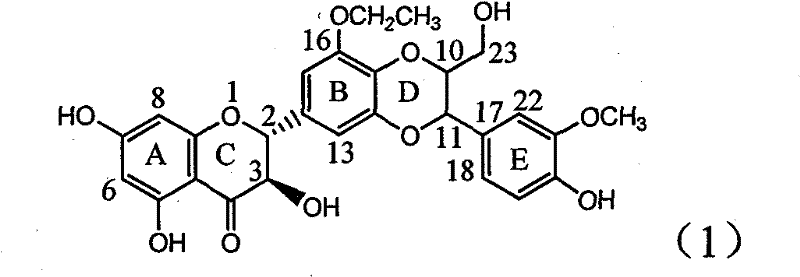
Application of ring E bromine substituted silybin in preparing medicaments for treating viral hepatitis B
InactiveCN101829094AInhibitory activityInhibition of replicative activityOrganic active ingredientsAntiviralsDiseasePositive control
The invention relates to application of ring E bromine substituted silybin in preparing medicaments for treating viral hepatitis B, in particular to application of a compound of a formula (1) and a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away hepatitis B surface antigens (HBsAg) and hepatitis e antigens (HBeAg) and suppressing the HBV (Hepatitis B Virus) DNA replication. The compound has definite activity on suppressing the HBsAg and the HBeAg, and in the presence of a concentration of 100 micrograms / milliliter, the intensities of the compound for clearing away the HBsAg and the HBeAg are respectively 38.2 percent and 39.1 percent which are respectively 2.4 times and 2.3 times of that of a positive control medicament (10,000 units / milliliter of alpha-interferon). Meanwhile, in the presence of the concentration, the suppression ratio of the compound on the HBV DNA is 36 percent which is close to that of the alpha-interferon. Accordingly, the flavone lignan or the pharmaceutically acceptable salt thereof are indicated to be capable of being used for preparing non-nucleoside medicaments for clearing away the HBsAg and the HBeAg, suppressing the HBV DNA replication and treating HBV infection diseases.
Owner:DALI UNIV
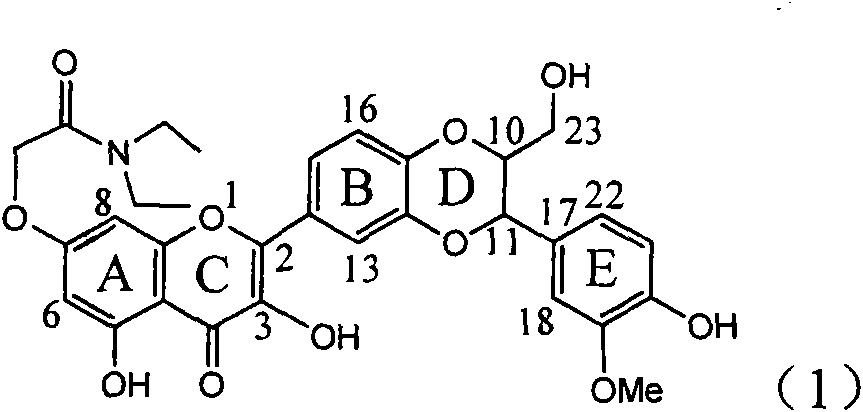
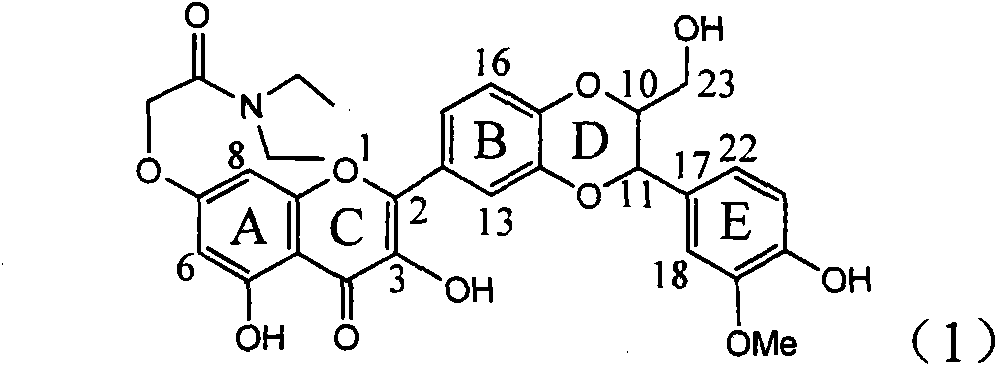
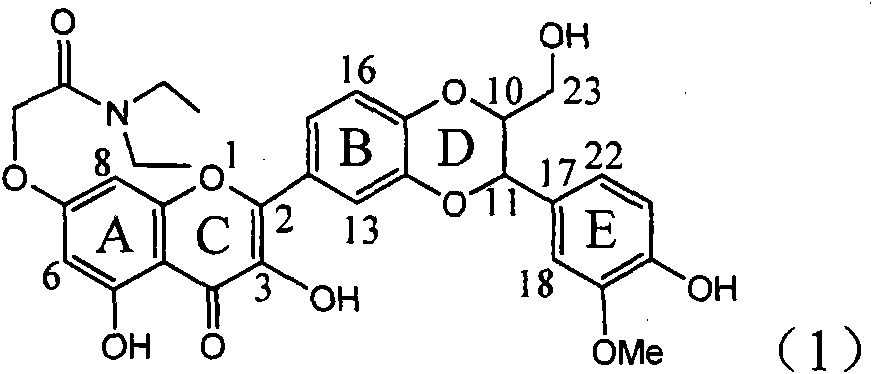
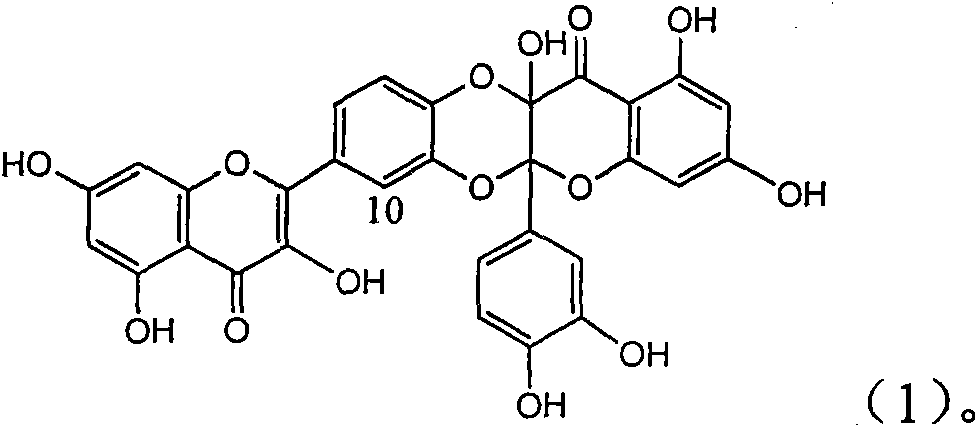
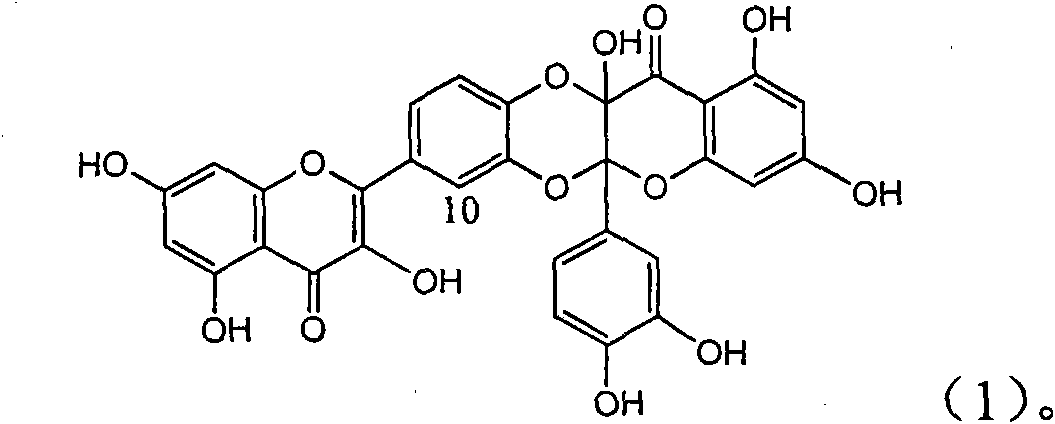
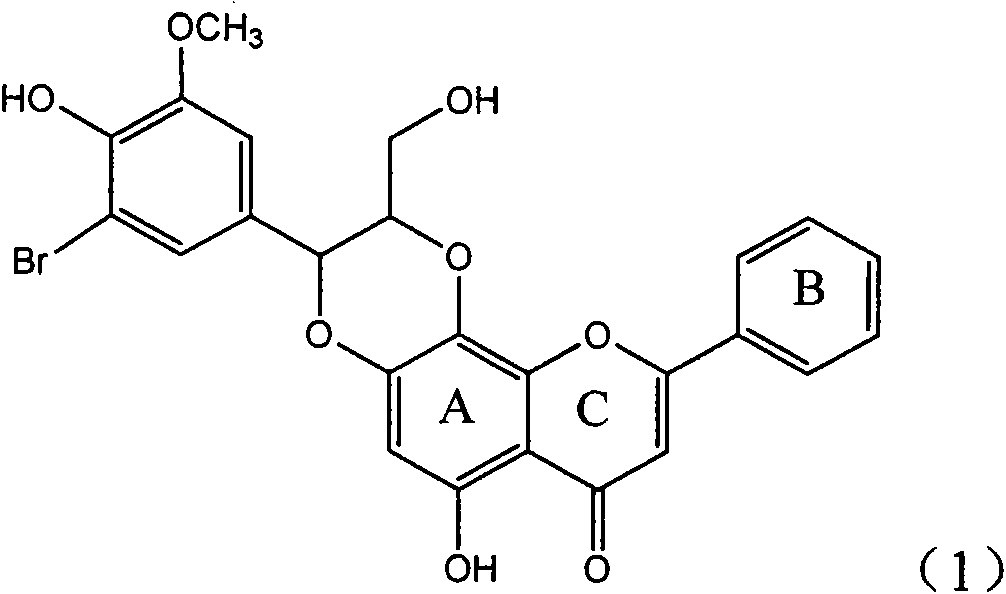
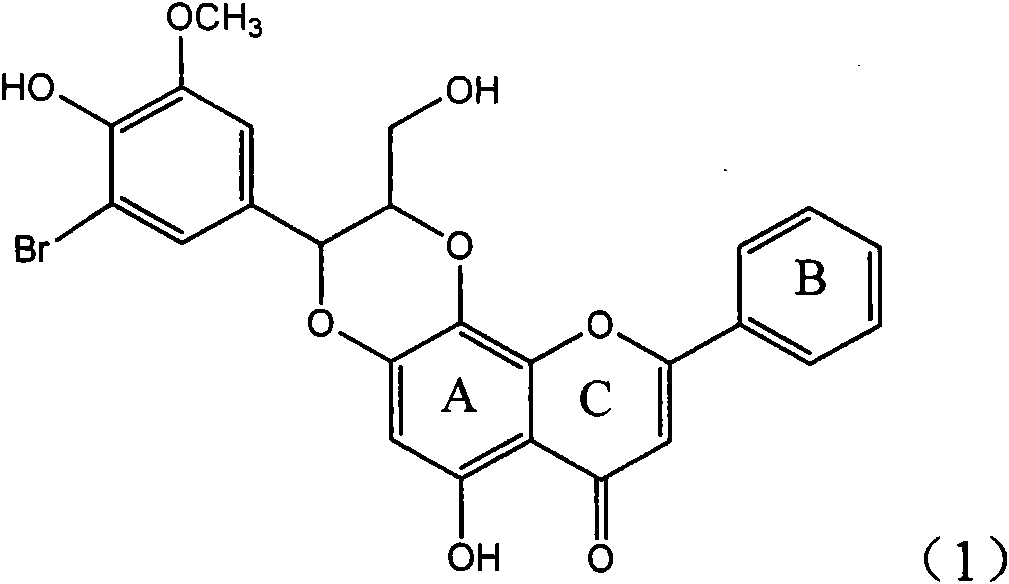
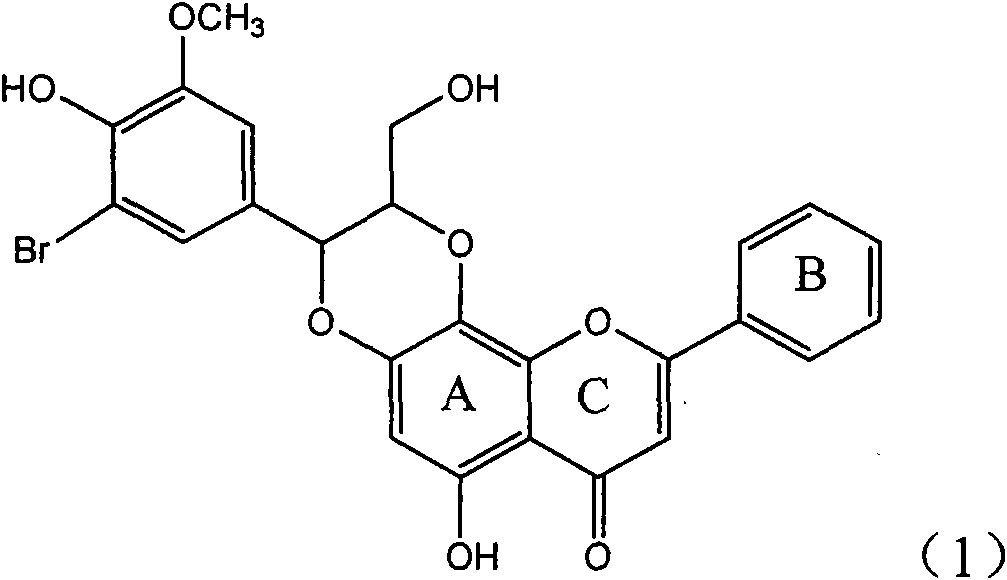
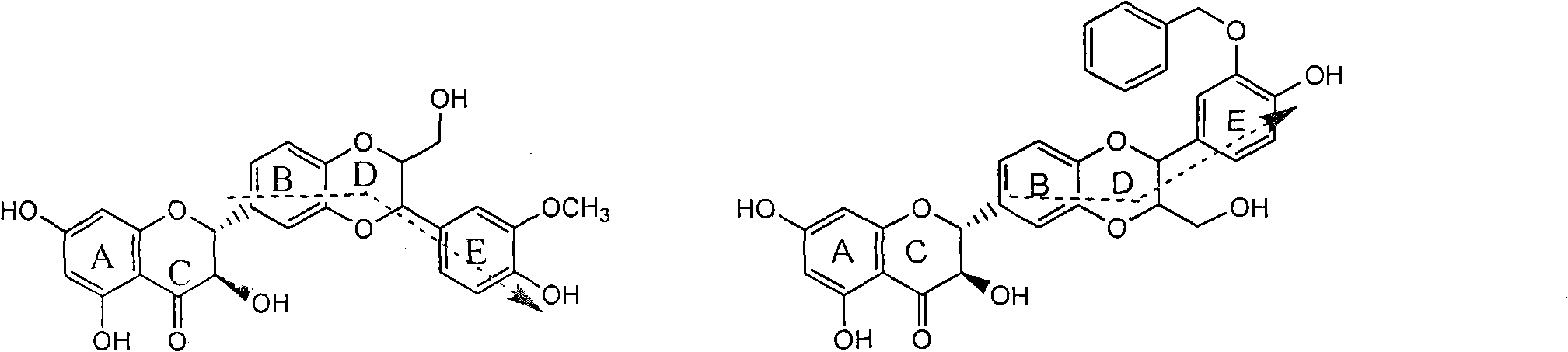
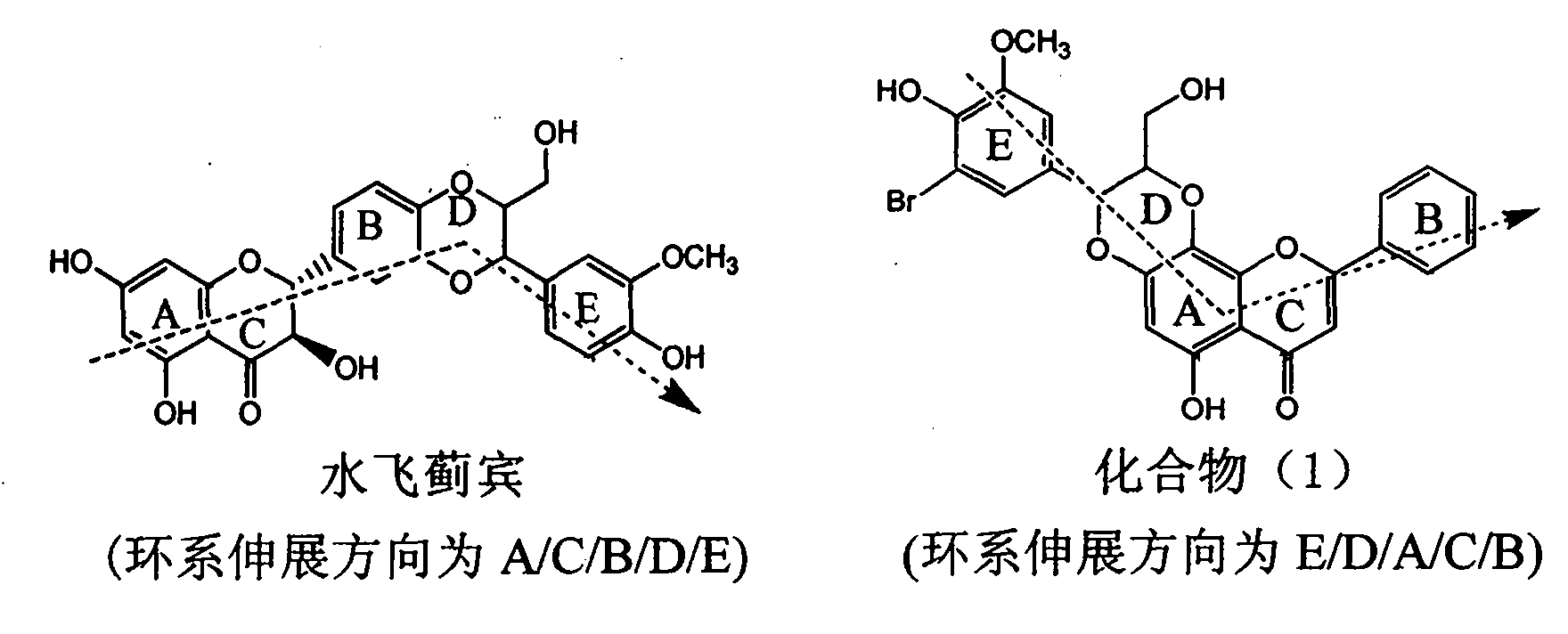
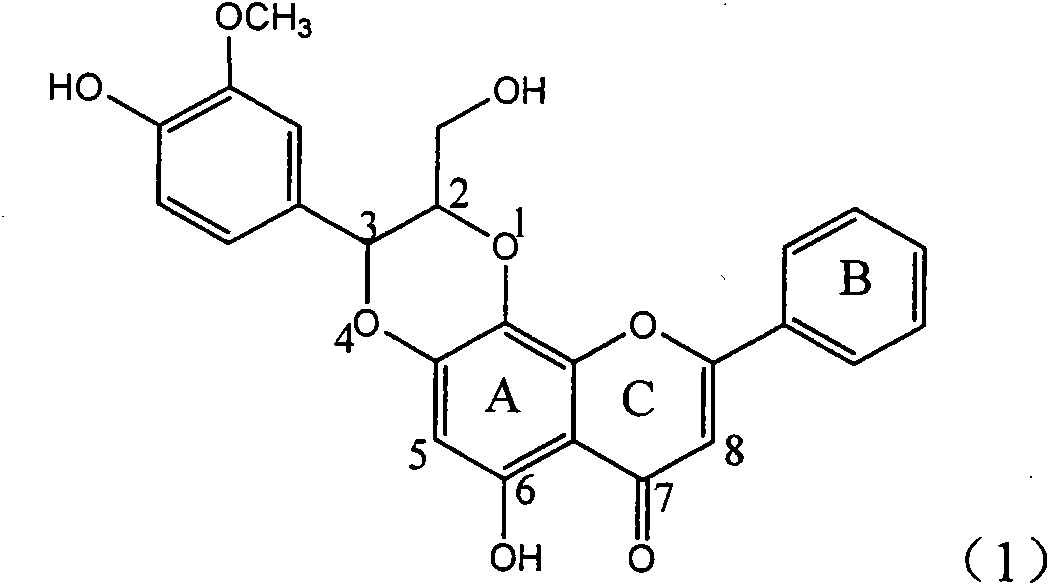
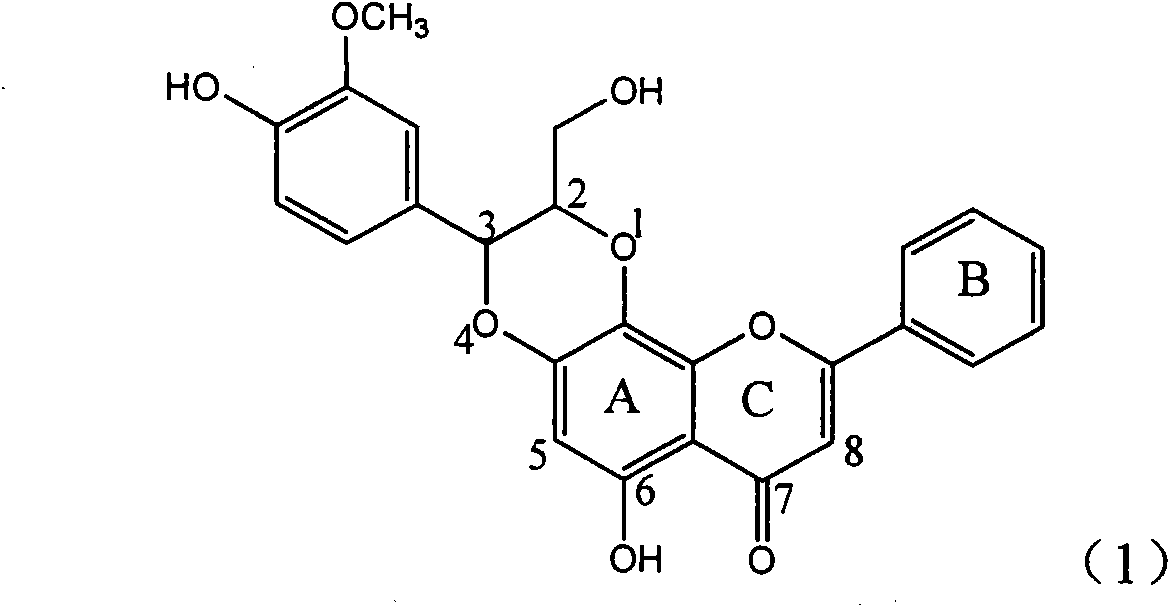
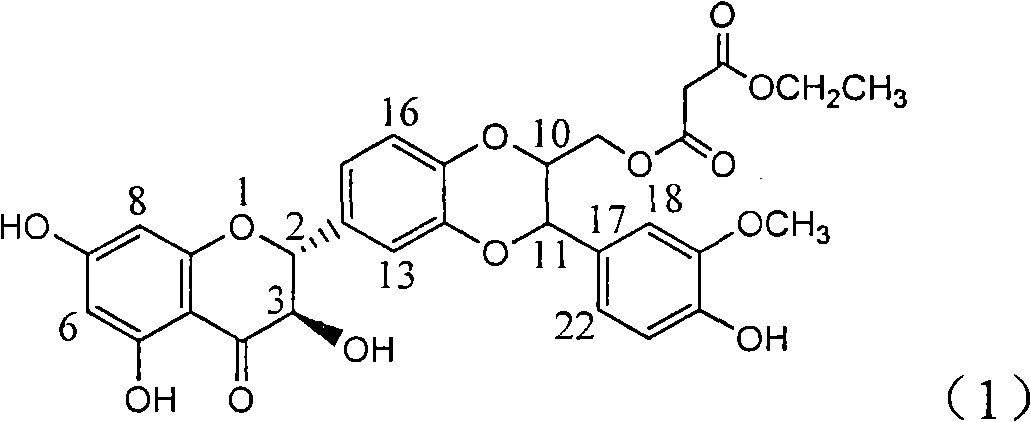
Application of ring A coupling flavonolignan in preparing medicaments for treating viral hepatitis B
InactiveCN101829104AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseasePositive control
The invention relates to application of ring A coupling flavonolignan in preparing medicaments for treating viral hepatitis B, in particular to application of a compound of the formula (1) or a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away HBsAg (Hepatitis B Surface Antigen) and HBeAg (Hepatitis B e Antigen) and suppressing the HBV (Hepatitis B Virus) DNA replication. The intensities of the flavonolignan for clearing away the HBsAg and the HBeAg are respectively 29.4 percent and 29.1 percent in the presence of a concentration of 20 micrograms / milliliter, which is respectively 1.8 times and 1.7 times of the corresponding activity of a positive control medicament (10,000 units / milliliter of alpha-interferon). What is even more exciting is that in the presence of the concentration, the suppression rate of the flavonolignan to the HBV DNA is higher than 83 percent, which is higher than that of Lamivudine which is a positive control and is 2.2 times of that of the alpha-interferon to the HBV DNA. Accordingly, the flavonolignan and the pharmaceutically acceptable salt thereof are indicated to be capable of being expected to be used for preparing non-nucleoside medicaments for clearing away the HBsAg and the HBeAg, suppressing the HBV DNA replication and treating HBV infection diseases.
Owner:DALI UNIV
Application of substituted isosilybin in preparing medicament for treating virus hepatitis B
InactiveCN101829098AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseasePositive control
The invention relates to application of substituted isosilybin in preparing a medicament for treating virus hepatitis B, in particular to application of E-ring substituted isosilybin or medicinal salt thereof in preparing a medicament for clearing hepatitis B e antigen, inhibiting HBV DNA replication and treating hepatitis B virus infected diseases. The E-ring substituted isosilybin has strong effect of inhibiting HBeAg activity, and the strength of the E-ring substituted isosilybin at the concentration of 100 micrograms per milliliter for clearing the HBeAg is 3.5 times that of a positive control front-line medicament (10,000 units per milliliter of alpha-interferon); and moreover, the compound at the concentration of 100 micrograms per milliliter has strong inhibiting rate (97.7 percent) on the HBV DNA. Pharmacodynamical results show that the E-ring substituted isosilybin or the medicinal salt thereof can be expected to be used for preparing the medicament for treating the hepatitis B virus infected diseases.
Owner:DALI UNIV
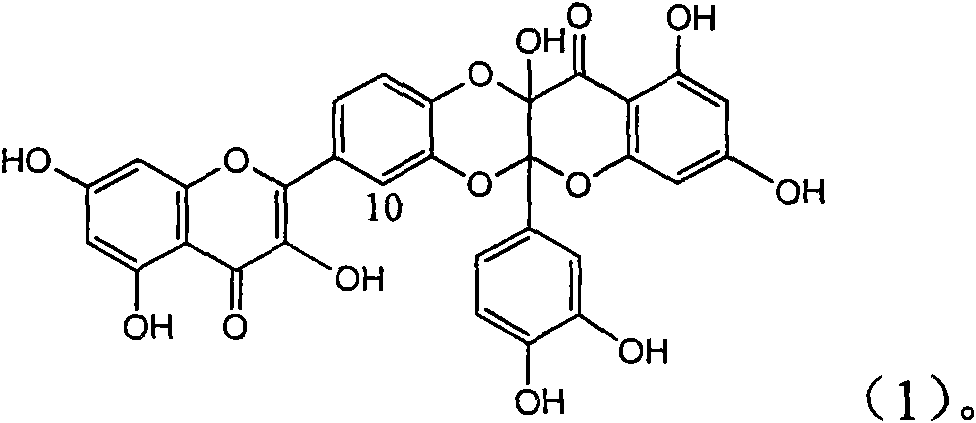
Application of flavone lignan (+/-) Scutellaprostin A in preparing medicaments for treating viral hepatitis type B
InactiveCN101953827AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseaseLignan
The invention relates to application of flavone lignan (+ / -) Scutellaprostin A in preparing medicaments for treating viral hepatitis type B, in particular to a compound with the formula (1) or pharmaceutically-acceptable salts thereof for preparing medicaments for clearing HBsAg and HBeAg and suppressing HBV (Hepatitis B Virus) DNA replication. In the invention, the intensities of the compound for clearing the HBsAg and the HBeAg under the concentration of 20 micrograms / milliliter respectively reach 81.8 percent and 81.9 percent, which are respectively 5.1 times and 4.8 times as high as the corresponding activity of alpha-interferon used as a positive contrast medicament; and what is more exciting, when the compound has the concentration, the compound performs a suppression ratio higher than 81 percent, and the value is also higher than that of both lamivudine and alpha-interferon. Accordingly, the flavone lignan or the pharmaceutically-acceptable salts can be expectably used for preparing nucleoside medicaments for clearing the HBsAg and the HBeAg, suppressing the HBV DNA replication and treating HBV infected diseases.
Owner:DALI UNIV
Application of E-ring demethoxy-silibinin for preparing medicament for treating viral hepatitis B
InactiveCN101912383AConvenient sourceThe source is easy to getOrganic active ingredientsDigestive systemDiseasePositive control
The invention relates to application of E-ring demethoxy-silibinin for preparing medicaments for treating viral hepatitis B, and particularly to application of compound in formula (1) and pharmaceutically acceptable salt thereof for preparing medicaments for clearing HBsAg and HBeAg and suppressing HBV DNA replication. The invention has extremely superactive activity for suppressing the HBsAg and HBeAg; in the presence of the concentration of 20 microgram per millilitre, the intensities for clearing the HBsAg and HBeAg are 95.0% and 34.4% respectively, which are 5.9 and 2.0 times corresponding activity of a positive control medicament alpha-interferon; and it should be noticed that the suppression ratio of the medicament for HBV DNA at the concentration is about 91.5%, which is 13% higher than lamivudine and 2.4 times alpha-interferon suppression activity. In summary, the flavonolignans or pharmaceutically acceptable salt thereof can be prospectively used for preparing non-nucleoside medicaments for clearing the HBsAg and HBeAg, suppressing HBV DNA replication and treating hepatitis B virus infection disease.
Owner:DALI UNIV
Application of diallyl propyl flavonolignan in preparation of medicament for curing hepatitis B
InactiveCN101829106ALower hepatitis B e antigenInhibition of HBV DNA replicationOrganic active ingredientsOrganic chemistryViral hepatitis bHenipavirus Infections
The invention relates to application of diallyl propyl flavonolignan in preparation a medicament for curing hepatitis B, in particular to application of a flavonolignan or pharmaceutically acceptable salts thereof in preparation of the medicament for curing the hepatitis B by clearing hepatitis B e-antigen and inhibiting HBV DNA replication. The flavonolignan has hepatitis B virus e-antigen (HBeAg) inhibiting activities, and has the inhibition strength higher than a positive control first-line medicament, namely lamivudine and alpha-interferon, at a low concentration of 20 mu g / ml; simultaneously, at the concentration of 5 mu g / ml, the compound has an inhibition rate of the HBV DNA of over 70 percent; and therefore, the flavonolignan can be expected for preparing the medicament for curing the hepatitis B virus infection diseases by clearing the hepatitis B e-antigen and inhibiting the HBV DNA replication.
Owner:DALI UNIV
Application of ring A substituted silybin ester in preparing medicaments for treating viral hepatitis B
InactiveCN101829101AConvenient sourceThe source is easy to getOrganic active ingredientsDigestive systemDiseasePositive control
The invention relates to application of ring A substituted silybin ester in preparing medicaments for treating viral hepatitis B, in particular to application of silybin ester flavonolignan substituted by ethoxycarbonyl methyl on the ring A or a pharmaceutically acceptable salt thereof for preparing medicaments for reducing the hepatitis B virus surface antigen (HBsAg), suppressing the HBV (Hepatitis B Virus) DNA replication and treating HBV infection diseases. The flavonolignan has quite obvious activity on suppressing the HBsAg, and in the presence of a concentration of 100 micrograms / milliliter, the intensity of the flavonolignan for clearing away the HBsAG exceeds that of alpha-interferon which is a positive control medicament by 3.3 times. Meanwhile, in the presence of a concentration of 20 micrograms / milliliter, suppression ratio of the compound to the HBV DNA is close to 60 percent. The pharmacodynamical results indicate that the flavonolignan or the pharmaceutically acceptable salt thereof can be expected to be used for preparing the medicaments for treating the HBV infection diseases.
Owner:DALI UNIV
Application of dimethyl dehydrated silybin in preparing medicaments for treating virus hepatitis B
InactiveCN101912385AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseaseNucleoside Reverse Transcriptase Inhibitor
The invention relates to application of dimethyl dehydrated silybin in preparing medicaments for treating virus hepatitis B, in particular to the application of 7 and 20-position methyl substituted dehydrated silybin or pharmaceutically acceptable salts thereof in preparing medicaments for removing HBsAG and HBsAg and medicaments for inhibiting HBV DNA replication. The dehydrated silybin has remarkably HBsAg and HBeAg inhibiting activity, wherein the strength for removing the HBsAg and the HBeAg at the concentration of 20 milligram / milliliter is 88.9 percent and 84.1 percent respectively, which are 5.5 times and 5.0 times that of a positive contrast medicament. More importantly, the dehydrated silybin shows the HBV DNA inhibition ratio of about 99.6 percent at the concentration of 20 milligram / milliliter, the activity exceeds lamivudine by 23 percent, which is 2.6 times that of interferon. Therefore, favonolignan or pharmaceutically acceptable salts thereof can be predictably used for preparing the non-nucleoside reverse transcriptase inhibitor medicaments for removing the HBsAg and HBeAg, inhibiting HBV DNA replication and treating hepatitis B virus infection diseases.
Owner:DALI UNIV
Application of dehydrogenation silybin in preparation of medicament for treating virus hepatitis B
InactiveCN101829097AConvenient sourceThe source is easy to getOrganic active ingredientsDigestive systemDiseasePositive control
The invention relates to application of dehydrogenation silybin in preparation of a medicament for treating virus hepatitis B, in particular to application of the dehydrogenation silybin or pharmaceutically acceptable salt thereof in preparation of the medicament for inhibiting HBV DNA replication. The medicament has obvious high hepatitis B virus desoxyribonucleic acid (HBV DNA) inhibiting activities and shows over 55 percent of inhibition rate for the HBV DAN at the concentration of 20 mg / ml, which is 1.5 times higher than that of a positive control medicament (10,000 unit / ml of alpha-interferon). The pharmacodynamics result shows the flavonolignan or the pharmaceutically acceptable salt thereof can be expected for preparing the non-nucleoside medicament for inhibiting HBV DNA replication and curing diseases caused by infection of hepatitis B virus.
Owner:DALI UNIV
Application of B/E ring substituted silybin for preparing medicament for treating virus hepatitis B
InactiveCN101919840AInhibition of HBV DNA replicationGood economic and social benefitsOrganic active ingredientsOrganic chemistryMicrogramHenipavirus Infections
The invention relates to application of a B / E ring substituted silybin for preparing a medicament for treating virus hepatitis B, in particular to the application of a B ring ethoxy and E ring methoxy substituted silybin ester or medicinal salts thereof for preparing the medicament for eliminating hepatitis B virus surface antigens and hepatitis B e antigen and inhibiting HBV and DNA replication. The medicament has accurate activity of inhibiting HBsAg and HbeAg, and respective intensity of 18.5% and 23.0% in eliminating HBsAg and HbeAg at the concentration of 100 microgram / ml, which exceeds the positive control medicament of an alpha-interferon; and meanwhile, 23.3% inhibition ratio on HBV and DNA is displayed at the concentration of 100 microgram / ml; and therefore, flavonoid lignans or a medicinal salt thereof are proved to have the efficacy of inhibiting HbsAg, HbeAg, HBV and DNA. The application is predictably used for preparing the non-nucleoside medicament for eliminating HBsAg and HbeAg, inhibiting HBV and DNA replication and treating hepatitis B virus infectious diseases.
Owner:DALI UNIV
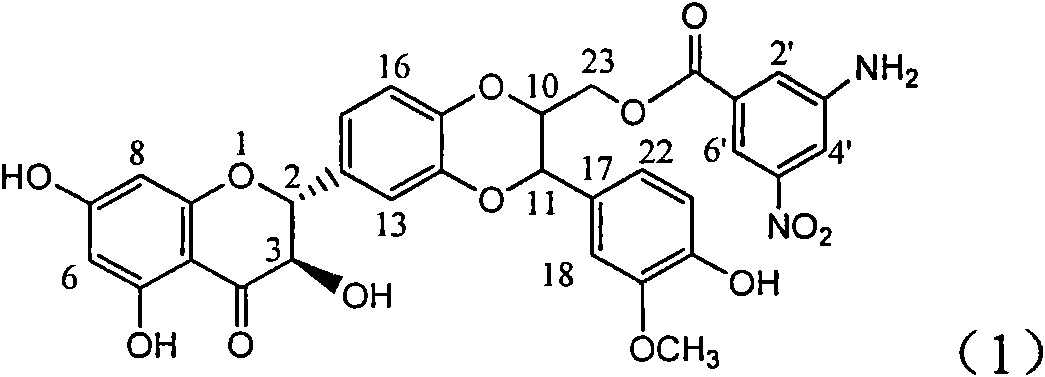
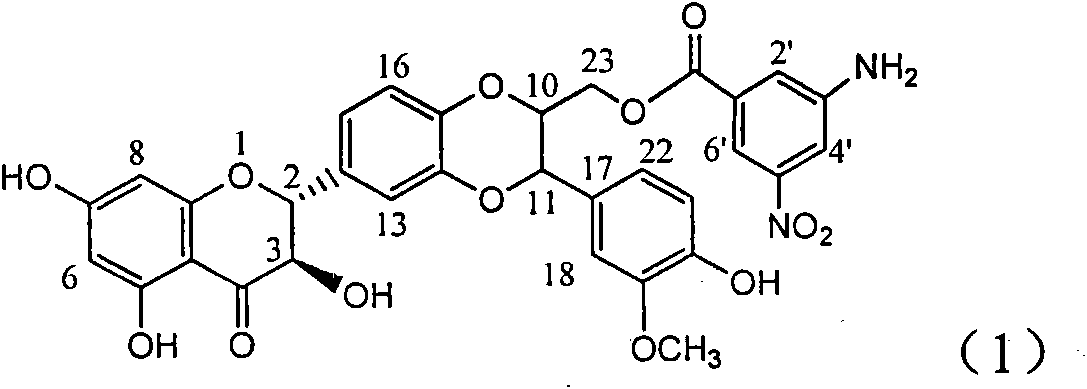
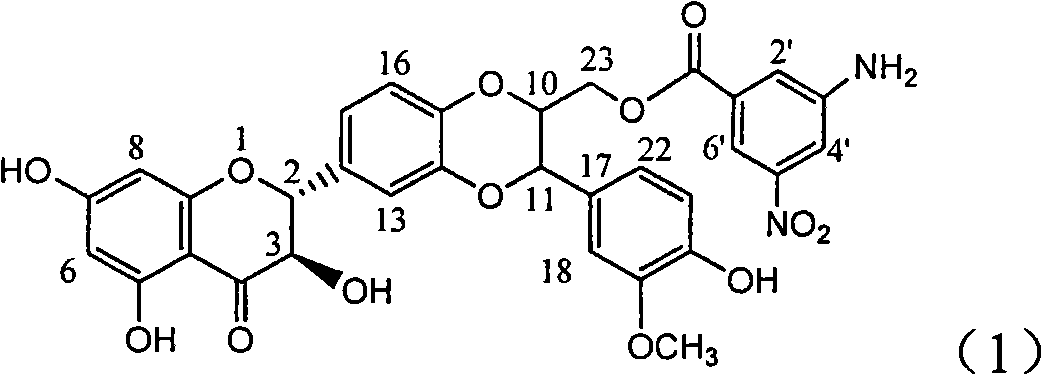
Application of aminobenzoyl silybin for preparing virus hepatitis B medicine
InactiveCN101829084AClears HBsAg and HBeAgInhibition of HBV DNA replicationOrganic active ingredientsAntiviralsHenipavirus InfectionsInfection disease
The invention relates to the application of aminobenzoyl silybin for preparing a virus hepatitis B medicine, in particular to the application of 23-bit p-aminobenzoyl substituted silybin ester or pharmaceutically acceptable salts thereof for preparing a medicine for eliminating hepatitis B virus surface antigen and hepatitis B e-antigen and inhibiting HBV DNA reproduction. The aminobenzoyl silybin has obvious activity for inhibiting HBsAg and HBeAg, the intensities for eliminating the HBsAg and the HBeAg are respectively of 73.9 percent and 95.3 percent when the concentration is 100 milligram / milliliter and are respectively higher than that of a positive control medicine alpha-interferon by 4.6 times and 5.6 times, and meanwhile, under the same concentration, the inhabitation ratio of the aminobenzoyl silybin for the HBV DNA is larger than 96 percent. The invention indicates the application of flavanolignan or the pharmaceutically acceptable salt which can be used for preparing a non-nucleoside medicine for eliminating the HBsAg and the HBeAg, inhibiting HBV DNA reproduction, treating hepatitis B virus infection diseases.
Owner:DALI UNIV
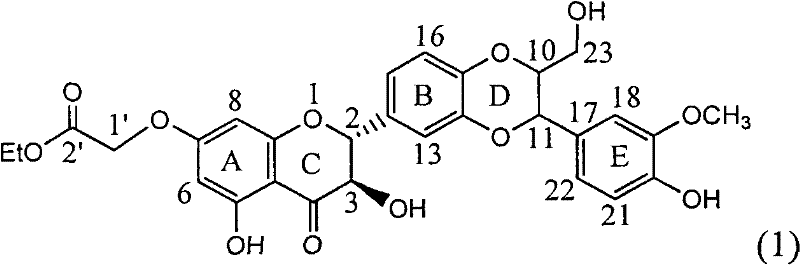
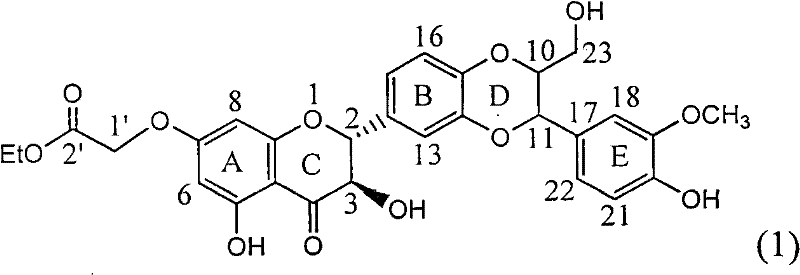
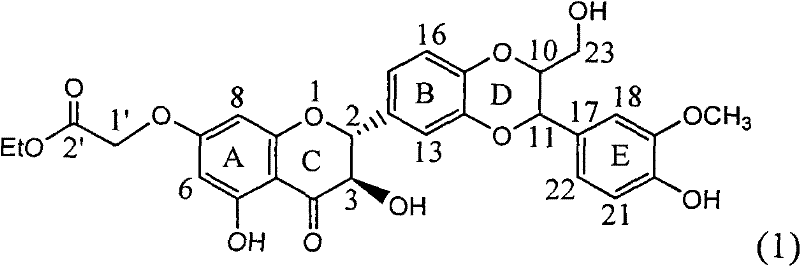
Application of substituted silybin ester in preparing medicament for treating virus hepatitis B
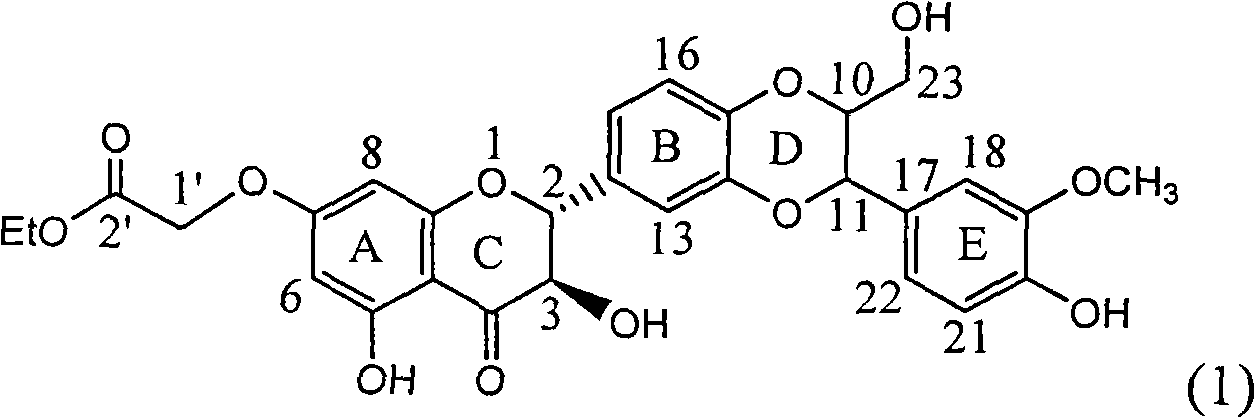
InactiveCN101829083AInhibition of replicationConvenient sourceOrganic active ingredientsAntiviralsDiseasePositive control
The invention relates to application of substituted silybin ester in preparing a medicament for treating virus hepatitis B, in particular to application of 23-position ethyl-malonyl substituted silybin ester or medicinal salt thereof in preparing a medicament for reducing hepatitis B virus surface antigen HBsAg, inhibiting HBV DNA replication and treating hepatitis B virus infected diseases. The flavonolignan has remarkable effect of inhibiting HBsAg activity, and the strength of the flavonolignan at the concentration of 100 micrograms per milliliter for clearing the HBsAg is two times and half that of a positive control medicament alpha-interferon; and meanwhile, the compound has strong inhibiting activity on the HBV DNA. Pharmacodynamical results show that the flavonolignan or the medicinal salt thereof can be expected to be used for preparing the medicament for reducing hepatitis B virus surface antigen, inhibiting HBV DNA replication and treating the hepatitis B virus infected diseases.
Owner:DALI UNIV
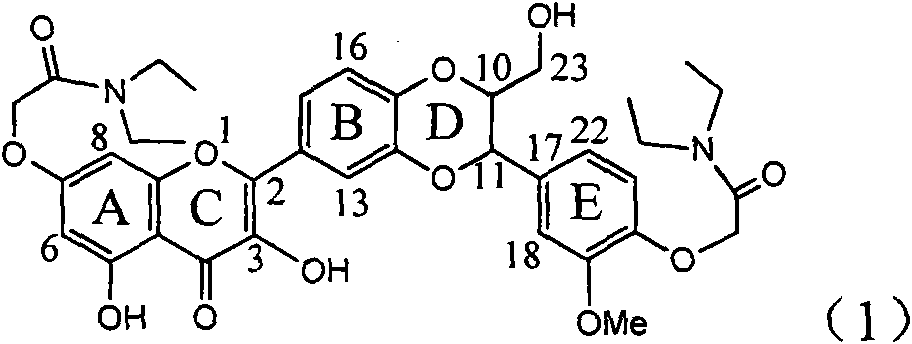
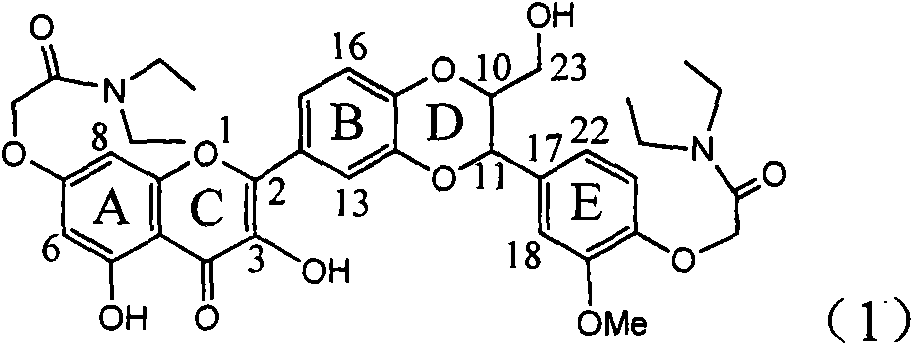
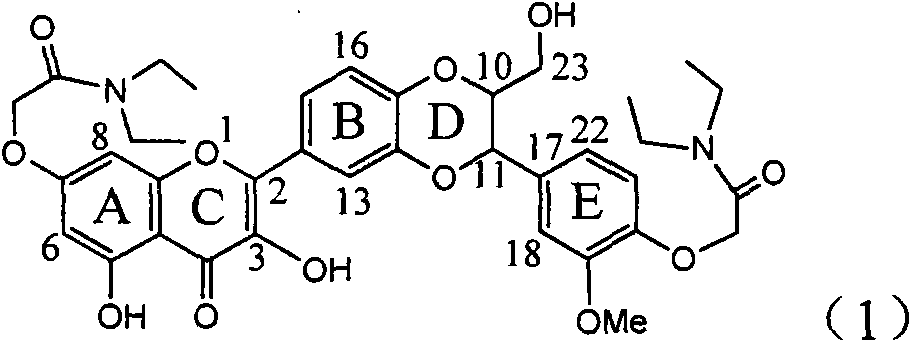
Application of diamine formyl dehydrogenated silybin serving as medicament for curing viral hepatitis B
InactiveCN101829090BConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsPositive controlHBsAg
The invention relates to application of diamine formyl dehydrogenated silybin serving as a medicament for curing viral hepatitis B, in particular to application of a flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents or pharmaceutically acceptable salts thereof in preparation of a medicament for clearing HBsAg and HBeAg and a medicament for inhibiting HBV DNA replication. The flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents has extremely high HBsAg and HBeAg inhibiting activities; when the flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents is at a concentration of 20 mu g / ml, the inhibition rates of the HBsAg and the HBeAg are respectively 94.4 percent and 95.7 percent which exceed 5.9 times and 5.7 times those of a positive control alpha-interferon; and simultaneously the inhibition rate of the HBV DNA is 99.7 percent when the flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents is at the same concentration, and the inhibition activity of the flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents is higher than that of lamivudine and the alpha-interferon. In summary, the flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents or the pharmaceutically acceptable salts thereof can be expected for preparing non-nucleoside medicaments for clearing the HBsAg and the HBeAg, inhibiting the HBV DNA replication, and curing the hepatitis B virus infection diseases.
Owner:DALI UNIV
Application of B-ring ethyoxyl flavanonol in preparing medicaments for treating hepatitis B viruses
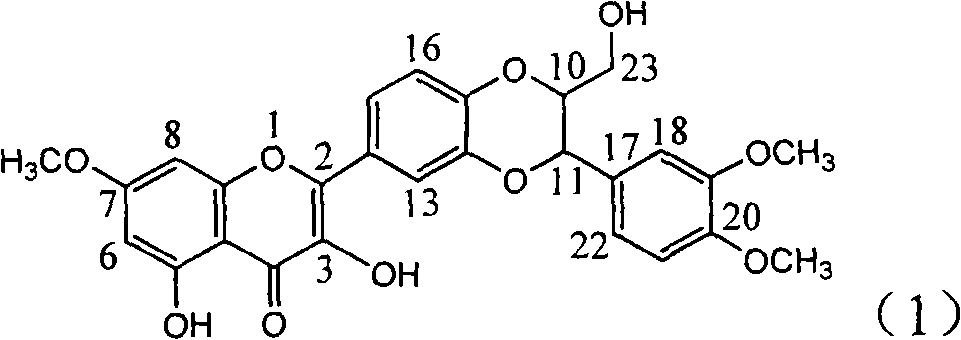
InactiveCN101822664BConvenient sourceThe source is easy to getOrganic active ingredientsOrganic chemistryDiseasePositive control
The invention relates to application of B-ring ethyoxyl flavanonol in preparing medicaments for treating hepatitis B viruses, in particular to application of a compound as shown in a formula (1) or a medicinal salt thereof in preparing medicaments for clearing away hepatitis B virus surface antigens (HBsAg) and hepatitis B e-antigen (HBeAg) and medicaments for inhibiting the duplication of hepatitis B virus desoxyribonucleic acid (HBV DNA). The compound or the medicinal salt thereof has extremely obvious activity on inhibiting the HBsAg and the HBeAg, and in the presence of a concentration of20 microgram / milliliter, the intensities for clearing away the HBsAg and the HBeAg of the compound or the medicinal salt thereof are respectively 99.8 percent and 48.5 percent and are 6.2 times and 2.7 times of that of alpha-interferon which is a positive control medicament. More importantly, in the presence of the concentration, the inhibition ratio of the compound or the medicinal salt thereof to the HBV DNA is 64.7 percent, and the activity is 1.7 times of that of the alpha-interferon. Accordingly, the flavone lignan or the medicinal salt thereof can be expected to be used for preparing non-nucleoside medicaments for treating infectious diseases of the hepatitis B viruses.
Owner:DALI UNIV
Application of ring E bromine substituted silybin in preparing medicaments for treating viral hepatitis B
InactiveCN101829094BInhibitory activityInhibition of replicative activityOrganic active ingredientsAntiviralsPositive controlHepatitis B Surface Antigens
The invention relates to application of ring E bromine substituted silybin in preparing medicaments for treating viral hepatitis B, in particular to application of a compound of a formula (1) and a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away hepatitis B surface antigens (HBsAg) and hepatitis e antigens (HBeAg) and suppressing the HBV (Hepatitis B Virus) DNA replication. The compound has definite activity on suppressing the HBsAg and the HBeAg, and in the presence of a concentration of 100 micrograms / milliliter, the intensities of the compound for clearing away the HBsAg and the HBeAg are respectively 38.2 percent and 39.1 percent which are respectively 2.4 times and 2.3 times of that of a positive control medicament (10,000 units / milliliter of alpha-interferon). Meanwhile, in the presence of the concentration, the suppression ratio of the compound on the HBV DNA is 36 percent which is close to that of the alpha-interferon. Accordingly, the flavone lignan or the pharmaceutically acceptable salt thereof are indicated to be capable of being used for preparing non-nucleoside medicaments for clearing away the HBsAg and the HBeAg, suppressing the HBV DNA replication and treating HBV infection diseases.
Owner:DALI UNIV
Application of flavone lignan (+/-) Scutellaprostin A in preparing medicaments for treating viral hepatitis type B
InactiveCN101953827BConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseaseLignan
The invention relates to application of flavone lignan (+ / -) Scutellaprostin A in preparing medicaments for treating viral hepatitis type B, in particular to a compound with the formula (1) or pharmaceutically-acceptable salts thereof for preparing medicaments for clearing HBsAg and HBeAg and suppressing HBV (Hepatitis B Virus) DNA replication. In the invention, the intensities of the compound for clearing the HBsAg and the HBeAg under the concentration of 20 micrograms / milliliter respectively reach 81.8 percent and 81.9 percent, which are respectively 5.1 times and 4.8 times as high as the corresponding activity of alpha-interferon used as a positive contrast medicament; and what is more exciting, when the compound has the concentration, the compound performs a suppression ratio higher than 81 percent, and the value is also higher than that of both lamivudine and alpha-interferon. Accordingly, the flavone lignan or the pharmaceutically-acceptable salts can be expectably used for preparing nucleoside medicaments for clearing the HBsAg and the HBeAg, suppressing the HBV DNA replication and treating HBV infected diseases.
Owner:DALI UNIV
Application of B/E ring substituted silybin for preparing medicament for treating virus hepatitis B
InactiveCN101919840BConvenient sourceThe source is easy to getOrganic active ingredientsOrganic chemistryPositive controlLignan
The invention relates to application of a B / E ring substituted silybin for preparing a medicament for treating virus hepatitis B, in particular to the application of a B ring ethoxy and E ring methoxy substituted silybin ester or medicinal salts thereof for preparing the medicament for eliminating hepatitis B virus surface antigens and hepatitis B e antigen and inhibiting HBV and DNA replication. The medicament has accurate activity of inhibiting HBsAg and HbeAg, and respective intensity of 18.5% and 23.0% in eliminating HBsAg and HbeAg at the concentration of 100 microgram / ml, which exceeds the positive control medicament of an alpha-interferon; and meanwhile, 23.3% inhibition ratio on HBV and DNA is displayed at the concentration of 100 microgram / ml; and therefore, flavonoid lignans or a medicinal salt thereof are proved to have the efficacy of inhibiting HbsAg, HbeAg, HBV and DNA. The application is predictably used for preparing the non-nucleoside medicament for eliminating HBsAg and HbeAg, inhibiting HBV and DNA replication and treating hepatitis B virus infectious diseases.
Owner:DALI UNIV
Application of methoxybenzene benzoyl silibinin in preparing medicine for treating viral hepatitis B
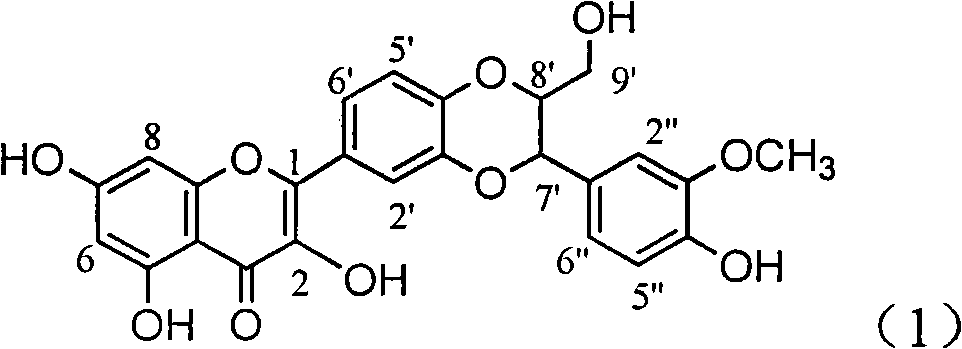
InactiveCN101829102AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsHigh concentrationDisease
The invention relates to an application of methoxybenzene benzoyl silibinin in preparing medicine for treating viral hepatitis B, in particular relates to an application of a silibinin esterified favonolignan compound substituted by 23-site 4-metoxybenzene benzoyl, or pharmaceutically acceptable salt thereof in preparing medicines for inhibiting HBV DNA replication, and treating hepatitis B virus infection diseases, wherein the favonolignan compound has exact inhibition activity to the hepatitis B virus, the inhibition activity thereof at 100 microgramme / mL to the HBV DNA replication of the hepatitis B virus is better than that of the alpha-interferon at the highest concentration of 10000 uint / mL, namely 47% higher than that of the inhibition activity of the alpha-interferon. The pharmacodynamics results show that the favonolignan compound or the pharmaceutically acceptable salt thereof can be expected to be used for preparing the medicine for inhibiting non-nucleoside HBV DNA replication or treating the hepatitis B virus infection diseases.
Owner:DALI UNIV
Use of acetamide dehydrogenation silibinin as medicament for treating viral hepatitis B
InactiveCN101829091BPowerful removalInhibitory activityOrganic active ingredientsDigestive systemDehydrogenationInterferon alpha
The invention relates to the use of acetamide dehydrogenation silibinin as a medicament for treating viral hepatitis B, in particular to the use of dehydrogenation silibinin esters flavonoid lignanoid replaced by A ring methoxy formyl amine or pharmaceutically acceptable salt as the medicament for eliminating HBsAg (hepatitis B surface antigen) and HBeAg (hepatitis Be antigen) and restraining copy of HBV DNA. The cetamide dehydrogenation silibinin can obviously restrain the HBsAg and HBeAg activity, and the strengths for eliminating the HBsAg and HBeAg are 90.5% and 63.6% at the concentration of 20 microgramme / milliter and are 5.6 times and 3.8 times more than positive contrast medicament alpha-interferon. Meanwhile, the restraining rate to the HBV DNA is 90.4% at the concentration, is 12% higher than lamivudine, and is 2.4 times more than a- interferon. Therefore, the flavonoid lignanoid or the pharmaceutically acceptable salt can be expected for treating hepatitis B virus infection as the non-nucleoside medicament.
Owner:DALI UNIV
Application of flavanonol lignanoid in preparing antiviral hepatitis B medicine
InactiveCN101829100BConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseasePositive control
The invention relates to application of flavanonol lignanoid in preparing an antiviral hepatitis B medicine, in particular to application of angle flavanolignan or pharmaceutically acceptable salts thereof in preparing a medicine which can be used for eliminating hepatitis B e antigen HBeAg, inhibiting HBV DNA replication and treating hepatitis B virus infection diseases. The flavanolignan has a certain HBeVg activity inhibition, the HBeAg elimination intensity of the flavanolignan is higher than that of a positive control first-line medicine as lamivudine in light concentration and is equivalent to that of alpha-interferon of 1000 unit / ml, and meanwhile, under the concentration of 20 microgram / ml, the flavanolignan displays an inhibition ratio which is larger than 45 percent for HBV DNA.The pharmacodynamics result indicates the application of the flavanolignan or the pharmaceutically acceptable salts thereof in preparing the medicine as expected for eliminating hepatitis B e antigen, inhibiting HBV DNA replication and treating hepatitis B virus infection diseases.
Owner:DALI UNIV
Application of ring B ethyoxyl silybin in preparing medicaments for treating viral hepatitis B
InactiveCN101829089BConvenient sourceThe source is easy to getOrganic active ingredientsDigestive systemPositive controlInterferon alpha
The invention relates to application of ring B ethyoxyl silybin in preparing medicaments for treating viral hepatitis B, in particular to application of ring B ethyoxyl substituted silybin ester or a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away HBsAG (Hepatitis B Surface Antigen) and HBeAg (Hepatitis B e Antigen) and suppressing the HBV (Hepatitis B Virus) DNA replication. The compound has strong activity on suppressing the HBsAG and the HBeAg, and in the presence of a concentration of 20 micrograms / milliliter, the intensities for clearing the HBsAg and the HBeAg are respectively 64.6 percent and 44.8 percent which are 4.0 times and 2.7 times of that of alpha-interferon which is a positive control medicament. In the presence of the concentration, the suppression rate of the compound on the HBV DNA is 58.1 percent which is 1.5 times of the corresponding activity of the alpha-interferon. Accordingly, the flavonolignan or the pharmaceutically acceptable salt thereof are indicated to simultaneously have strong efficacy on suppressing the HBsAg, the HBeAg and the HBV DNA and can be expected to be used for preparing non-nucleoside medicaments for treating HBV infection diseases.
Owner:DALI UNIV
Application of substituted benzoyl silybin in preparing medicament for treating virus hepatitis B
InactiveCN101829092AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseaseHigh concentration
The invention relates to application of substituted benzoyl silybin in preparing a medicament for treating virus hepatitis B, in particular to application of 3-amino-5-nitrobenzoyl substituted silybin ester type flavonolignan or medicinal salt thereof in preparing a medicament for inhibiting HBV DNA replication and treating hepatitis B virus infected diseases. The flavonolignan has the effect of definitely inhibiting HBV DNA activity, the activity of the flavonolignan in high dosage (100 micrograms per milliliter) for inhibiting the HBV DNA replication is 55 percent higher than the inhibiting activity of alpha-interferon at highest concentration of 10,000 units per milliliter, and the flavonolignan belongs to an effectual non-nucleoside natural product for inhibiting the hepatitis B virus. Pharmacodynamical results show that the flavonolignan or the medicinal salt thereof can be expected to be used for preparing the medicament for inhibiting HBV DNA replication and treating the hepatitis B virus infected diseases.
Owner:DALI UNIV
Application of ring A substituted silybin ester in preparing medicaments for treating viral hepatitis B
InactiveCN101829101BConvenient sourceThe source is easy to getOrganic active ingredientsDigestive systemPositive controlInterferon alpha
The invention relates to application of ring A substituted silybin ester in preparing medicaments for treating viral hepatitis B, in particular to application of silybin ester flavonolignan substituted by ethoxycarbonyl methyl on the ring A or a pharmaceutically acceptable salt thereof for preparing medicaments for reducing the hepatitis B virus surface antigen (HBsAg), suppressing the HBV (Hepatitis B Virus) DNA replication and treating HBV infection diseases. The flavonolignan has quite obvious activity on suppressing the HBsAg, and in the presence of a concentration of 100 micrograms / milliliter, the intensity of the flavonolignan for clearing away the HBsAG exceeds that of alpha-interferon which is a positive control medicament by 3.3 times. Meanwhile, in the presence of a concentration of 20 micrograms / milliliter, suppression ratio of the compound to the HBV DNA is close to 60 percent. The pharmacodynamical results indicate that the flavonolignan or the pharmaceutically acceptable salt thereof can be expected to be used for preparing the medicaments for treating the HBV infection diseases.
Owner:DALI UNIV
Application of E-ring demethoxy-silibinin for preparing medicament for treating viral hepatitis B
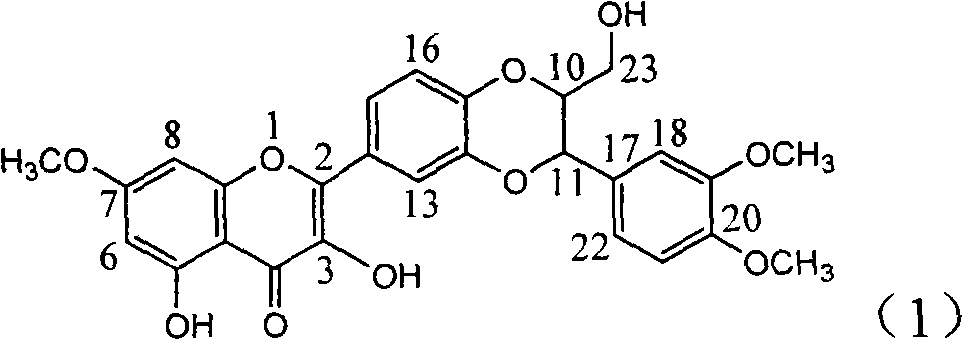
InactiveCN101912383BConvenient sourceThe source is easy to getOrganic active ingredientsDigestive systemDiseasePositive control
The invention relates to application of E-ring demethoxy-silibinin for preparing medicaments for treating viral hepatitis B, and particularly to application of compound in formula (1) and pharmaceutically acceptable salt thereof for preparing medicaments for clearing HBsAg and HBeAg and suppressing HBV DNA replication. The invention has extremely superactive activity for suppressing the HBsAg andHBeAg; in the presence of the concentration of 20 microgram per millilitre, the intensities for clearing the HBsAg and HBeAg are 95.0% and 34.4% respectively, which are 5.9 and 2.0 times corresponding activity of a positive control medicament alpha-interferon; and it should be noticed that the suppression ratio of the medicament for HBV DNA at the concentration is about 91.5%, which is 13% higher than lamivudine and 2.4 times alpha-interferon suppression activity. In summary, the flavonolignans or pharmaceutically acceptable salt thereof can be prospectively used for preparing non-nucleoside medicaments for clearing the HBsAg and HBeAg, suppressing HBV DNA replication and treating hepatitis B virus infection disease.
Owner:DALI UNIV
Application of B/E bi-methoxy silybin in preparing medicaments for treating viral hepatitis B
InactiveCN101829088BConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsPositive controlLignan
Owner:DALI UNIV
Application of benzo-phenylpropanoids in preparing drug for treating viral hepatitis B
InactiveCN101829093BConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsPhenylpropanoidHigh concentration
The invention relates to an application of benzo-phenylpropanoids in preparing drugs for treating viral hepatitis B, in particular to two benzo-phenylpropanoids or application of pharmaceutically acceptable salt thereof in preparing drugs for inhibiting the replication of hepatitis B virus desoxyribonucleic acid (HBV DNA) and treating hepatitis B virus infection diseases. The two benzo-phenylpropanoids definitely inhibit the activity of the HBV DNA, have the replication inhibition activity on the HBV DNA at high dose (100 microgrammes / milliliter) of 1.3-2.2 times higher than the inhibition activity at the highest concentration (10000 units / milliliter) of an alpha-interferon and belong to an efficient non-nucleoside natural product inhibiting the hepatitis B viruses; pharmacodynamics results show the application of the benzo-phenylpropanoids or the pharmaceutically acceptable salt thereof capable of preparing the drugs for inhibiting the replication of the HBV DNA and treating the hepatitis B virus infection diseases in anticipation.
Owner:DALI UNIV
Application of substituted isosilybin in preparing medicament for treating virus hepatitis B
InactiveCN101829098BConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseasePositive control
The invention relates to application of substituted isosilybin in preparing a medicament for treating virus hepatitis B, in particular to application of E-ring substituted isosilybin or medicinal salt thereof in preparing a medicament for clearing hepatitis B e antigen, inhibiting HBV DNA replication and treating hepatitis B virus infected diseases. The E-ring substituted isosilybin has strong effect of inhibiting HBeAg activity, and the strength of the E-ring substituted isosilybin at the concentration of 100 micrograms per milliliter for clearing the HBeAg is 3.5 times that of a positive control front-line medicament (10,000 units per milliliter of alpha-interferon); and moreover, the compound at the concentration of 100 micrograms per milliliter has strong inhibiting rate (97.7 percent) on the HBV DNA. Pharmacodynamical results show that the E-ring substituted isosilybin or the medicinal salt thereof can be expected to be used for preparing the medicament for treating the hepatitisB virus infected diseases.
Owner:DALI UNIV
Application of benzyl-containing flavonoid lignan in preparation of medicament for treating hepatitis B
InactiveCN101912387BConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsAntigenDisease
The invention relates to application of benzyl-containing flavonoid lignan in preparation of a medicament for treating hepatitis B, in particular to application of flavonoid lignan and pharmaceutically-acceptable salt thereof in preparation of the medicament for eliminating hepatitis B e antigen, inhibiting hepatitis B virus desoxyribonucleic acid (HBV DNA) replication and treating viral hepatitis B. The flavonoid lignan can inhibit the activity of hepatitis B virus e antigen (HBeAg); the inhibition intensity of the flavonoid lignan under low concentration of 20 micrograms per milliliter is much higher than that of a positive control front-line medicament lamivudine and an interferon; and the compound with the concentration of 20 micrograms per milliliter displays an inhabitation ratio over 50 percent on the HBV DNA, so that the application of the flavonoid lignan in preparation of the medicament for eliminating the hepatitis B e antigen and the medicament for inhibiting the HBV DNA replication and treating hepatitis B viral diseases can be anticipated.
Owner:DALI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com