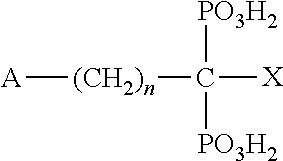Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
37 results about "Cathepsin C" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
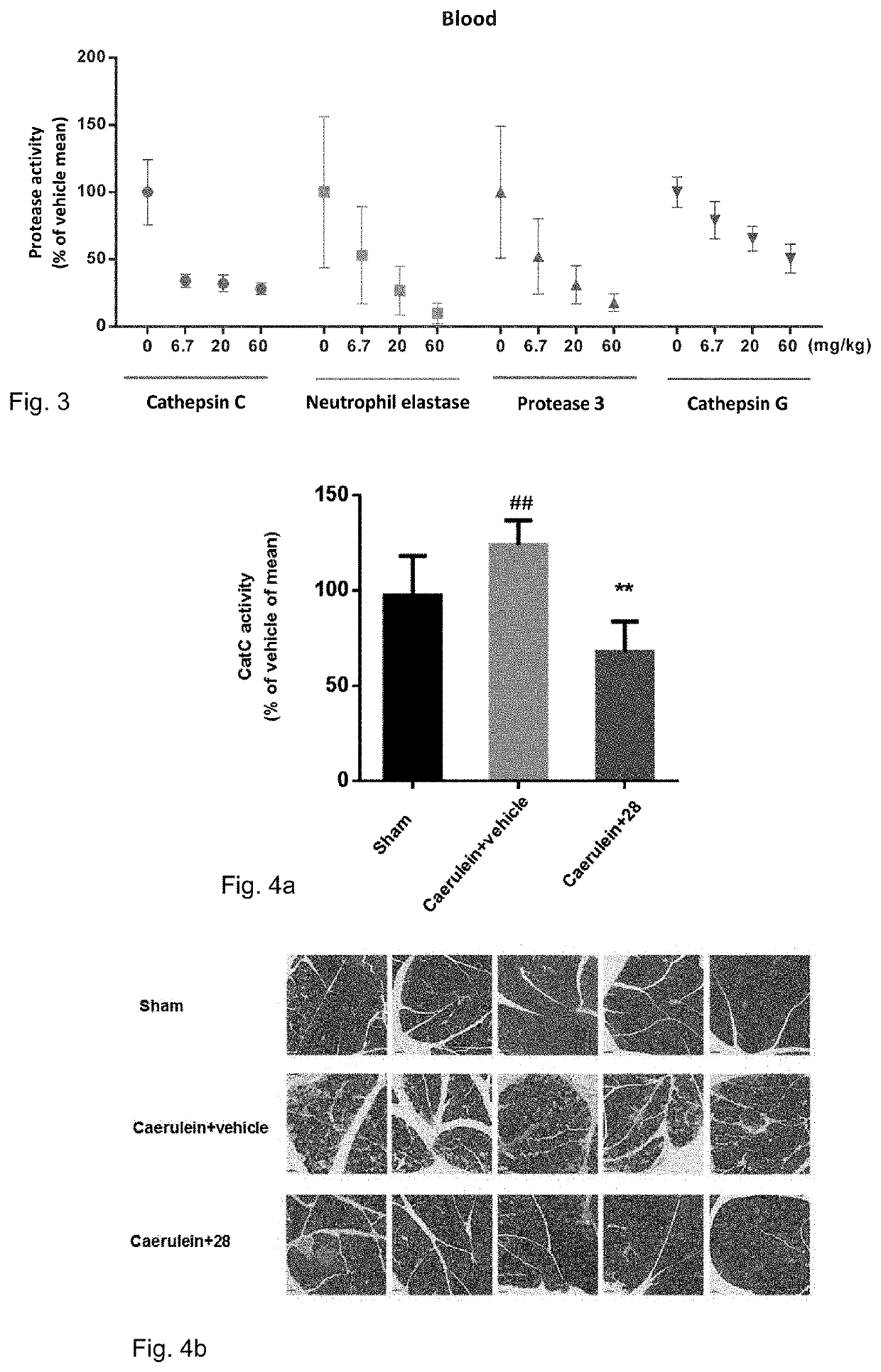
Cathepsin C (CTSC) also known as dipeptidyl peptidase I (DPP-I) is a lysosomal exo-cysteine protease belonging to the peptidase C1 family. In humans, it is encoded by the CTSC gene.
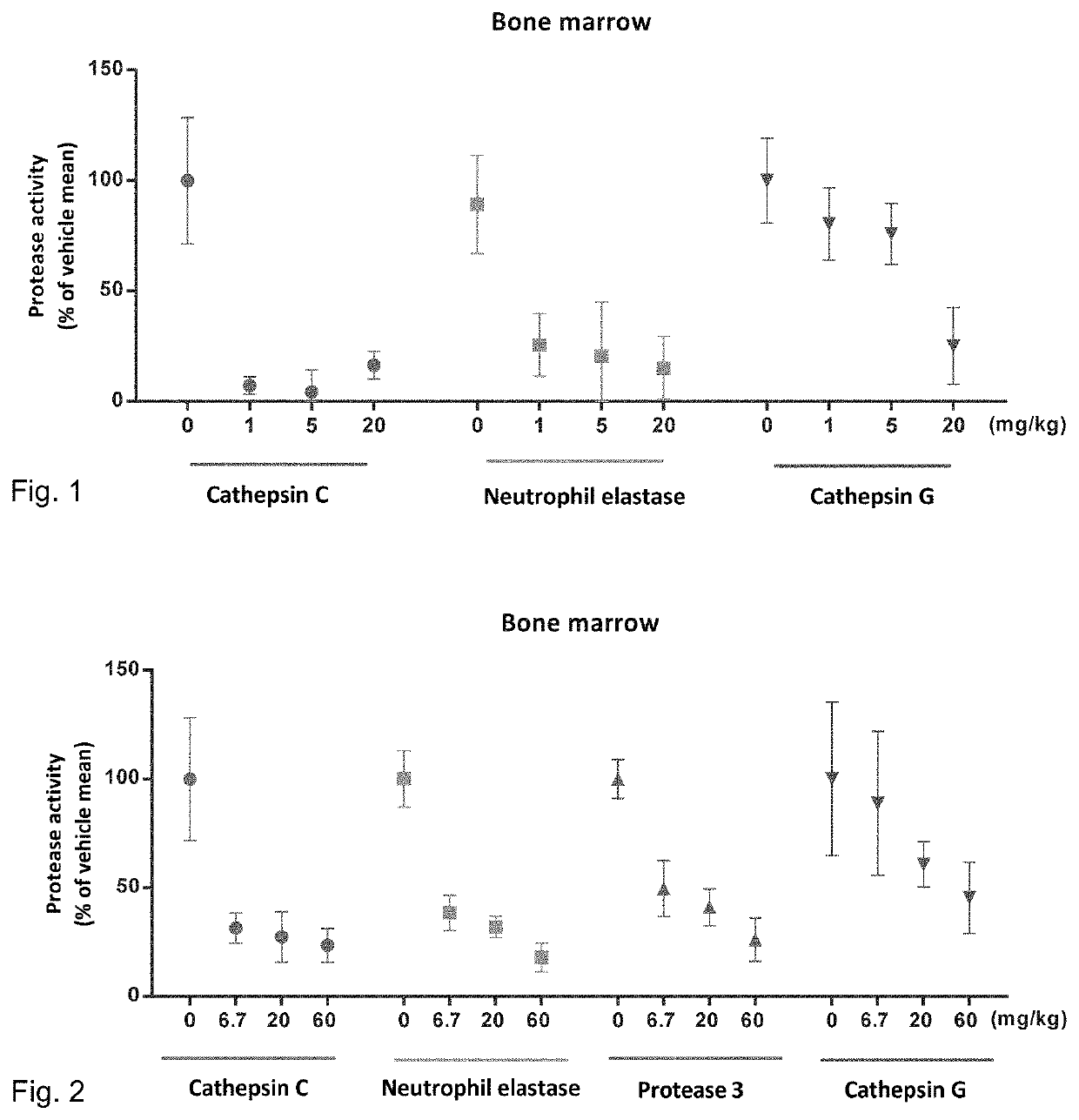
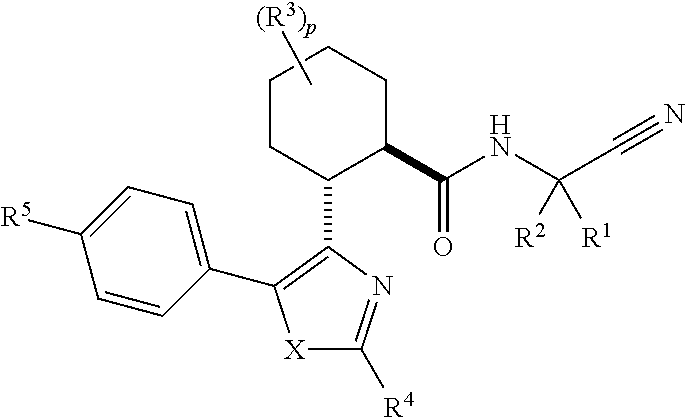
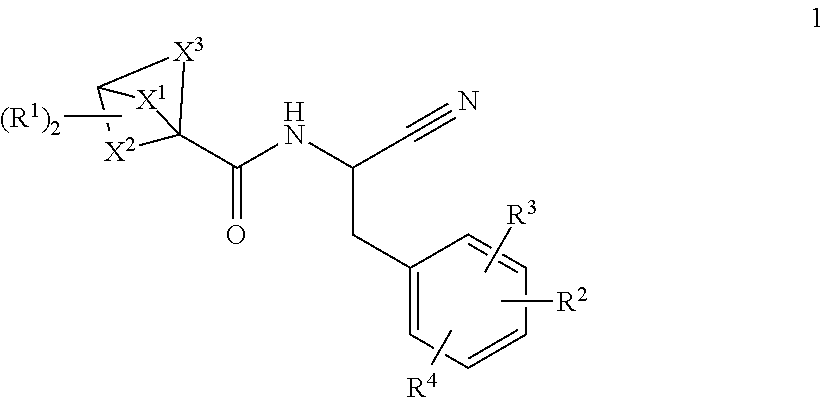
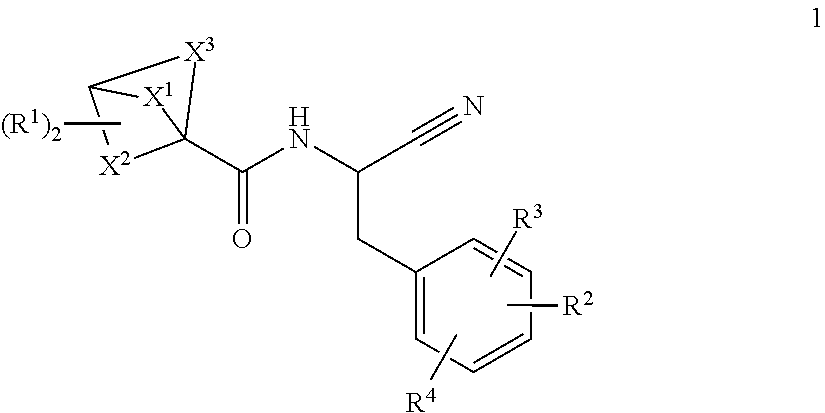
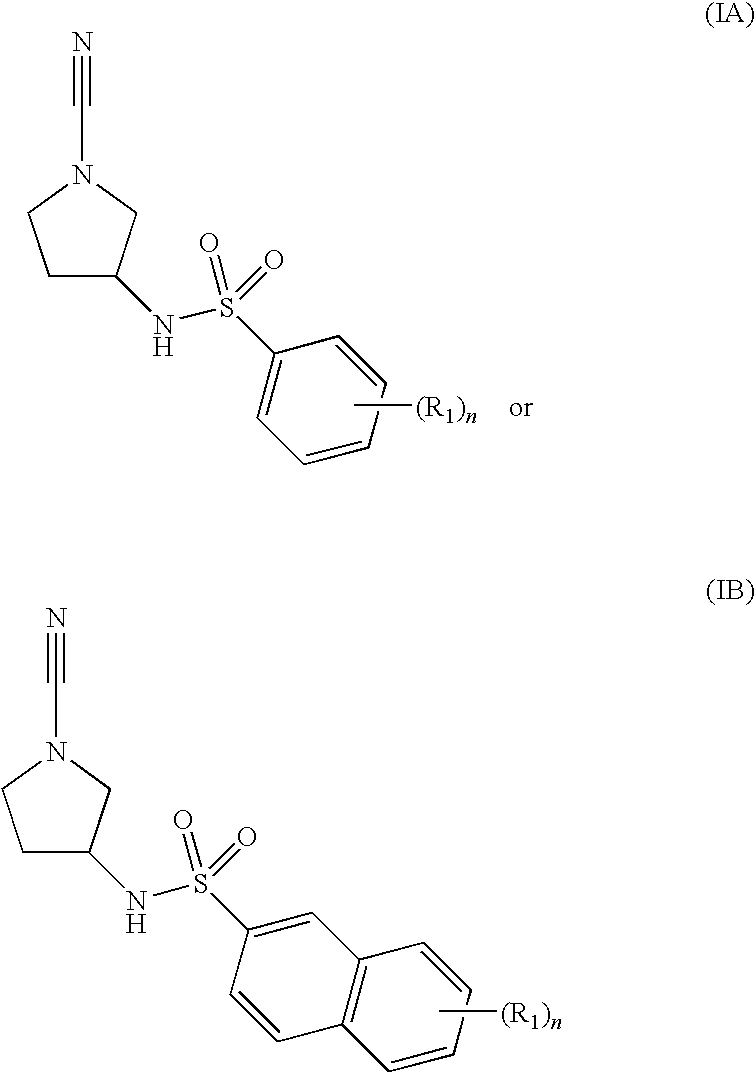
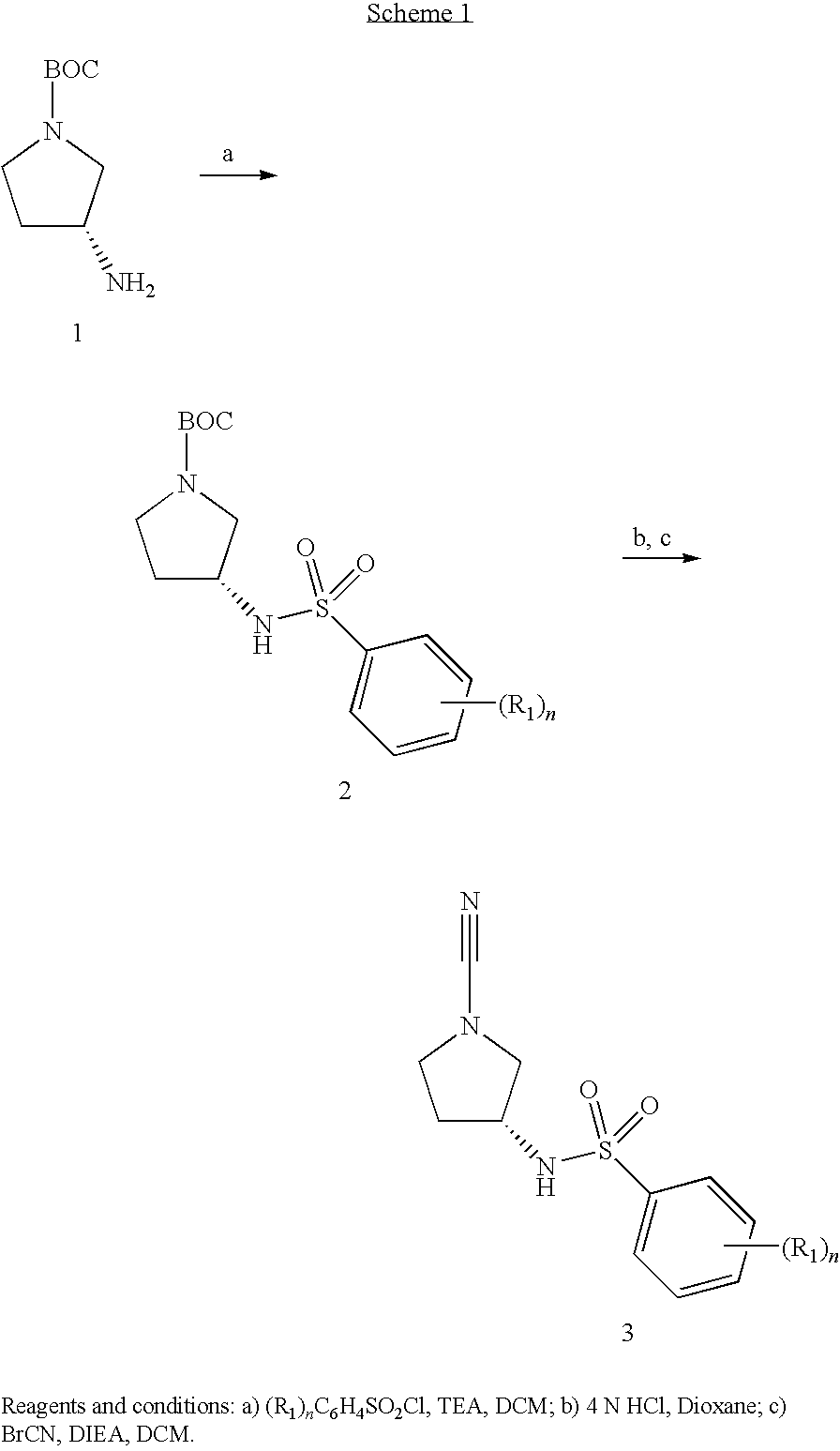
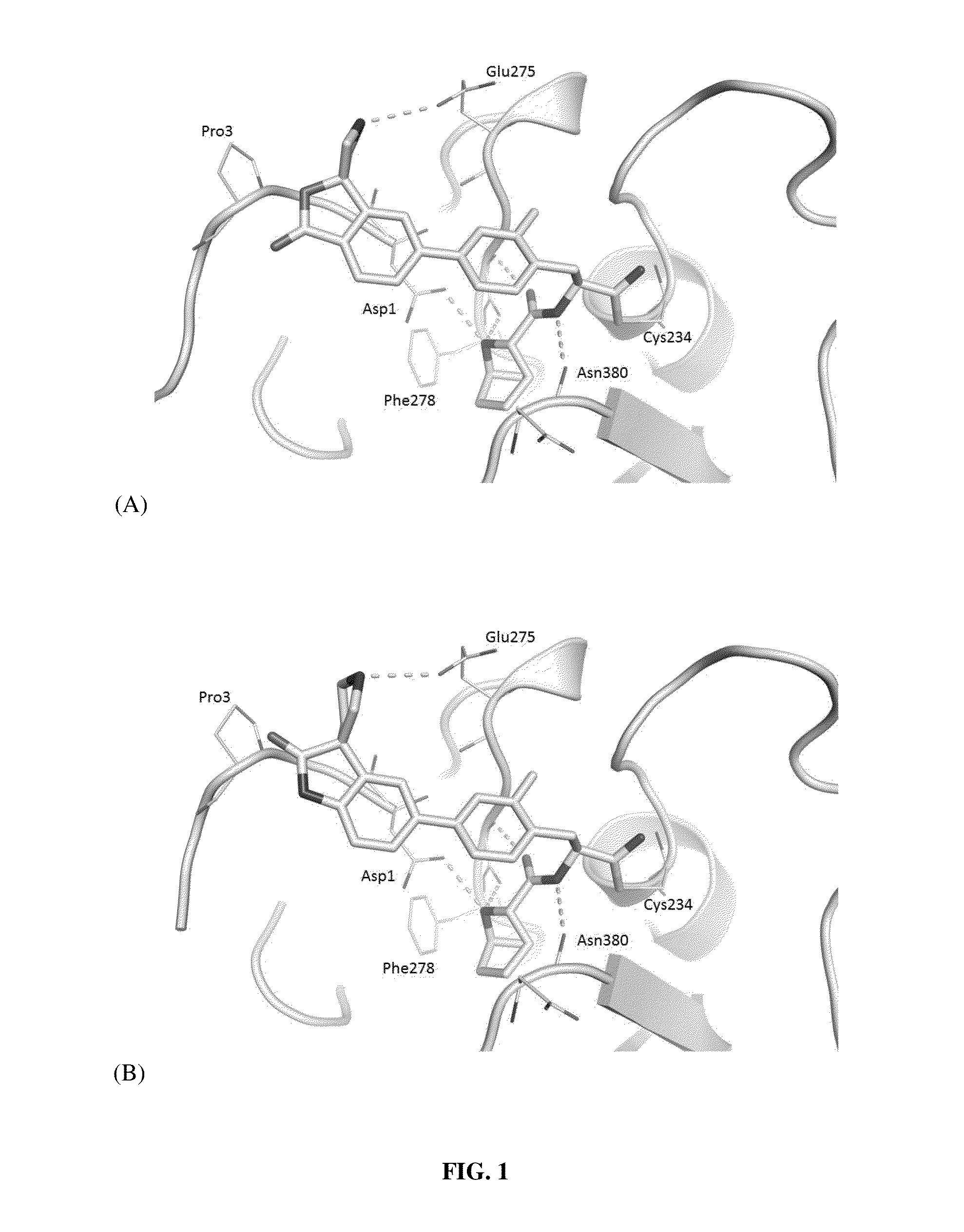
Substituted N- [1-cyano-2- (phenyl) ethyl] -2-azabicyclo [2.2.1] heptane-3-carboxamide inhibitors of cathepsin C
ActiveUS8999975B2Potent Cathepsin C activityHigh selectivityBiocideOrganic chemistryCathepsin CCombinatorial chemistry
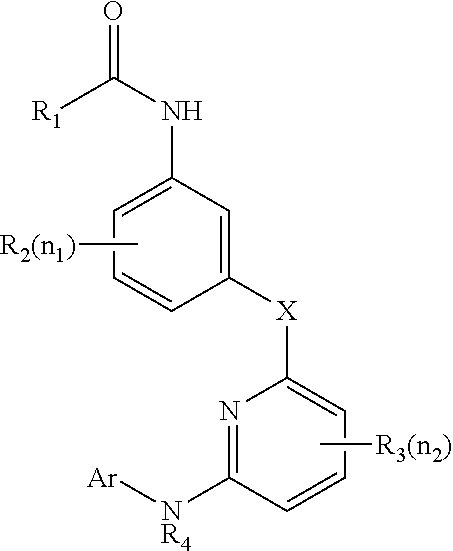
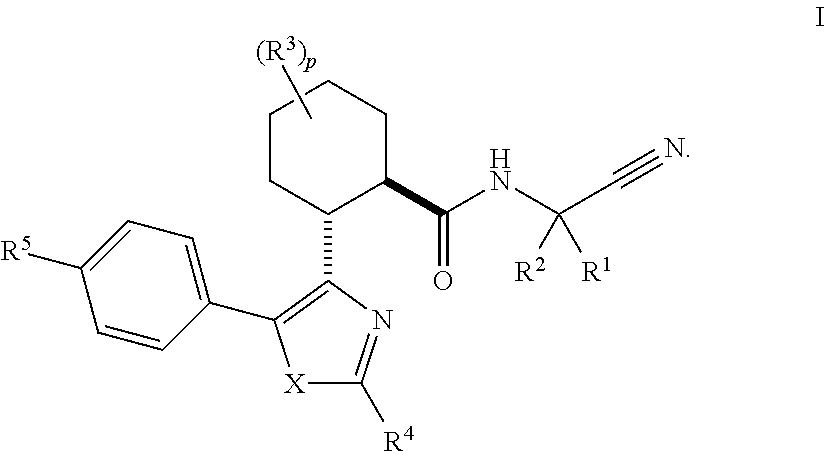
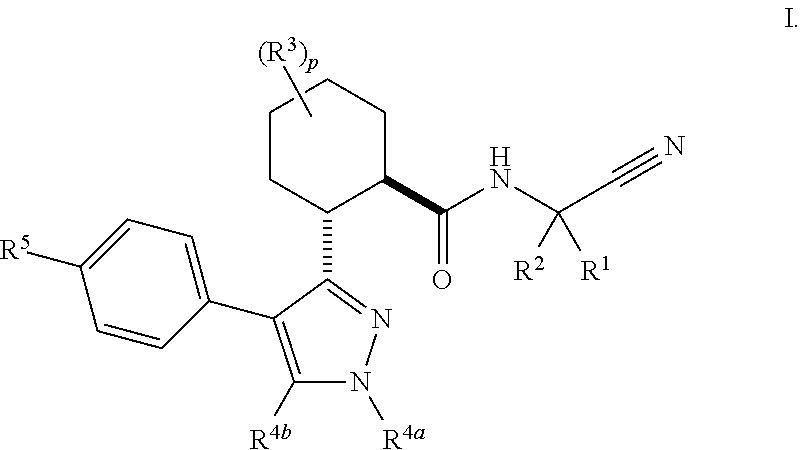
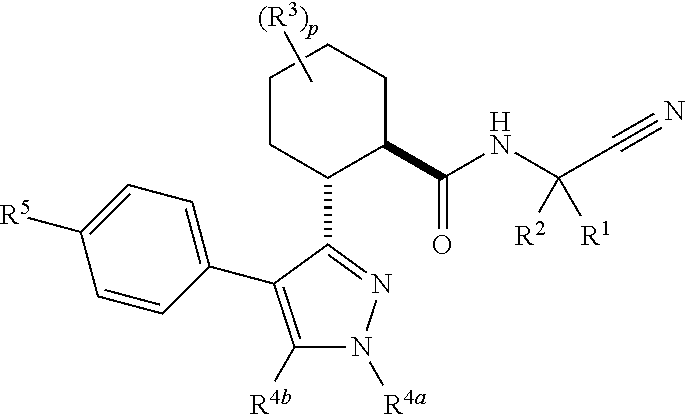
The present invention is directed to compounds of Formula I:wherein R1, R2 and n are described herein. These compounds and their pharmaceutically acceptable salts thereof are inhibitors of Cathepsin C.
Owner:BOEHRINGER INGELHEIM INT GMBH
Substituted bicyclic 1-carboxylic-acid (benzyl-cyano-methyl)-amides inhibitors of cathepsin C
Owner:BOEHRINGER INGELHEIM INT GMBH
Substituted 2-aza-bicyclo[2.2.2]octane-3-carboxylic acid (benzyl-cyano-methyl)-amides inhibitors of cathepsin C
Owner:BOEHRINGER INGELHEIM INT GMBH
Substituted oxetanes and their use as inhibitors of cathepsin C
This invention relates to a compound of formula Iand their use as inhibitors of Cathepsin C, pharmaceutical compositions containing the same, and methods of using the same as agents for treatment and / or prevention of diseases connected with dipeptidyl peptidase I activity, e.g. respiratory diseases.
Owner:BOEHRINGER INGELHEIM INT GMBH
Substituted 2-aza-bicyclo[2.2.1]heptane-3-carboxylic acid (cyano-methyl)-amides inhibitors of cathepsin C
Owner:BOEHRINGER INGELHEIM INT GMBH
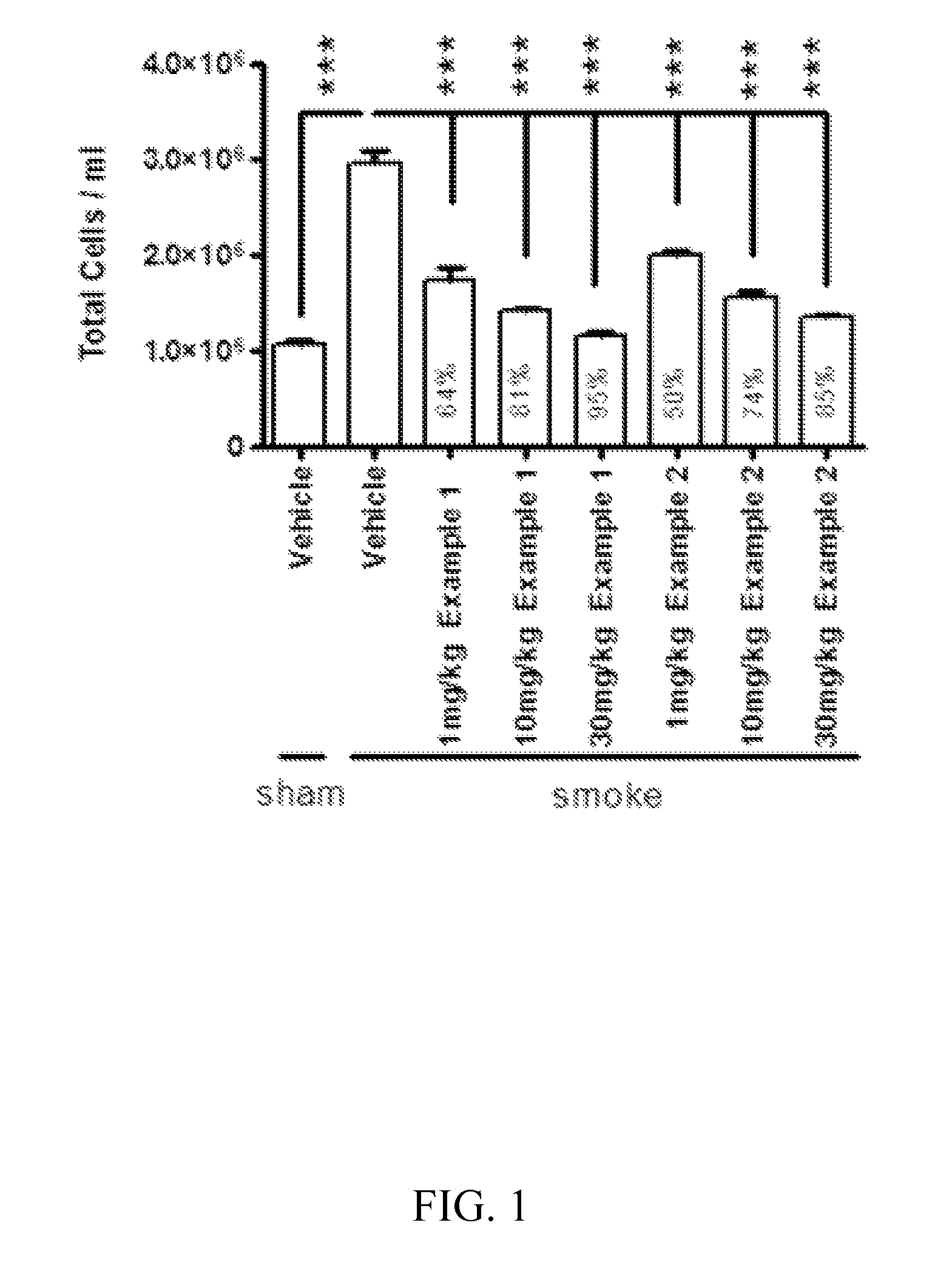
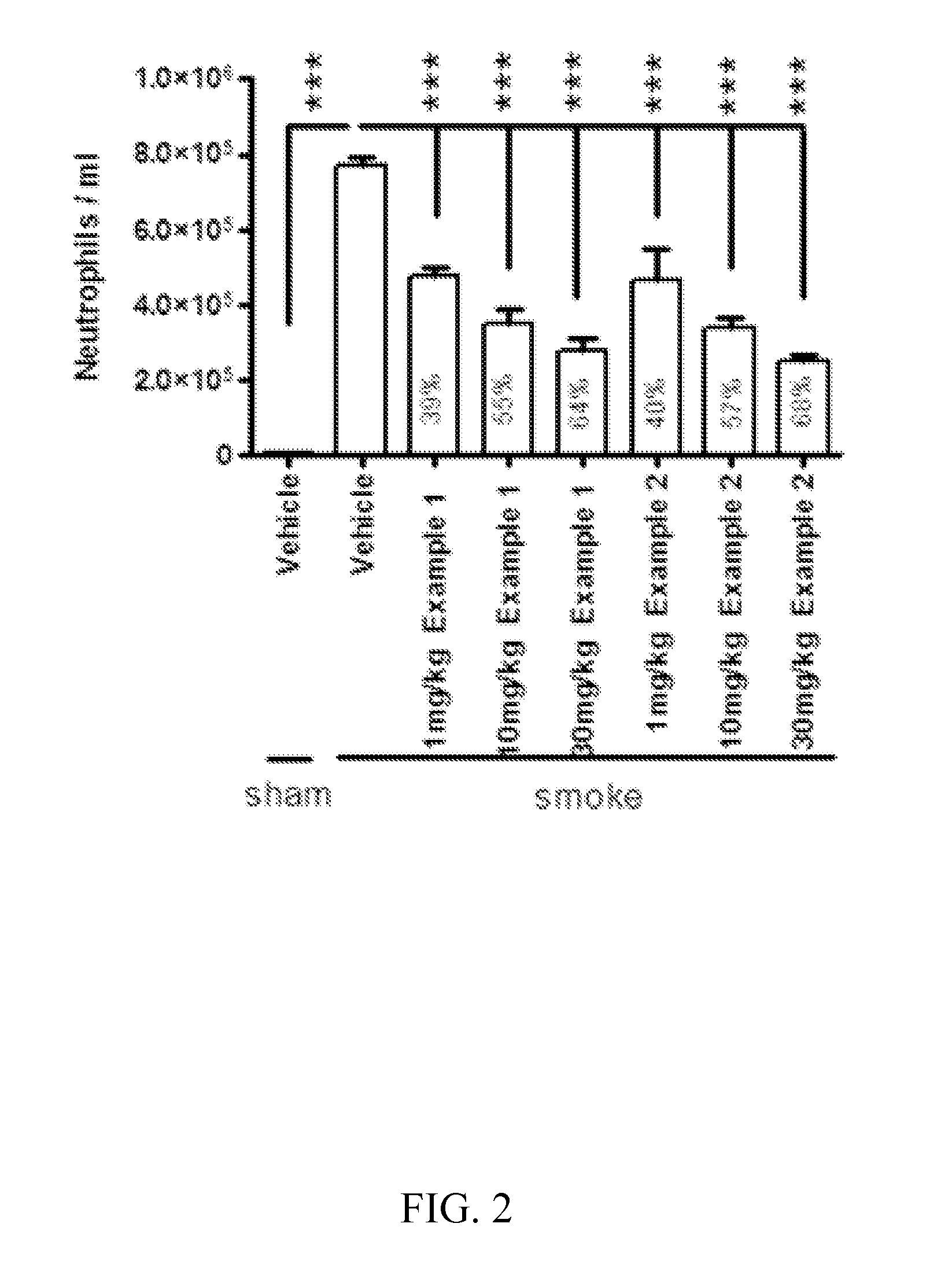
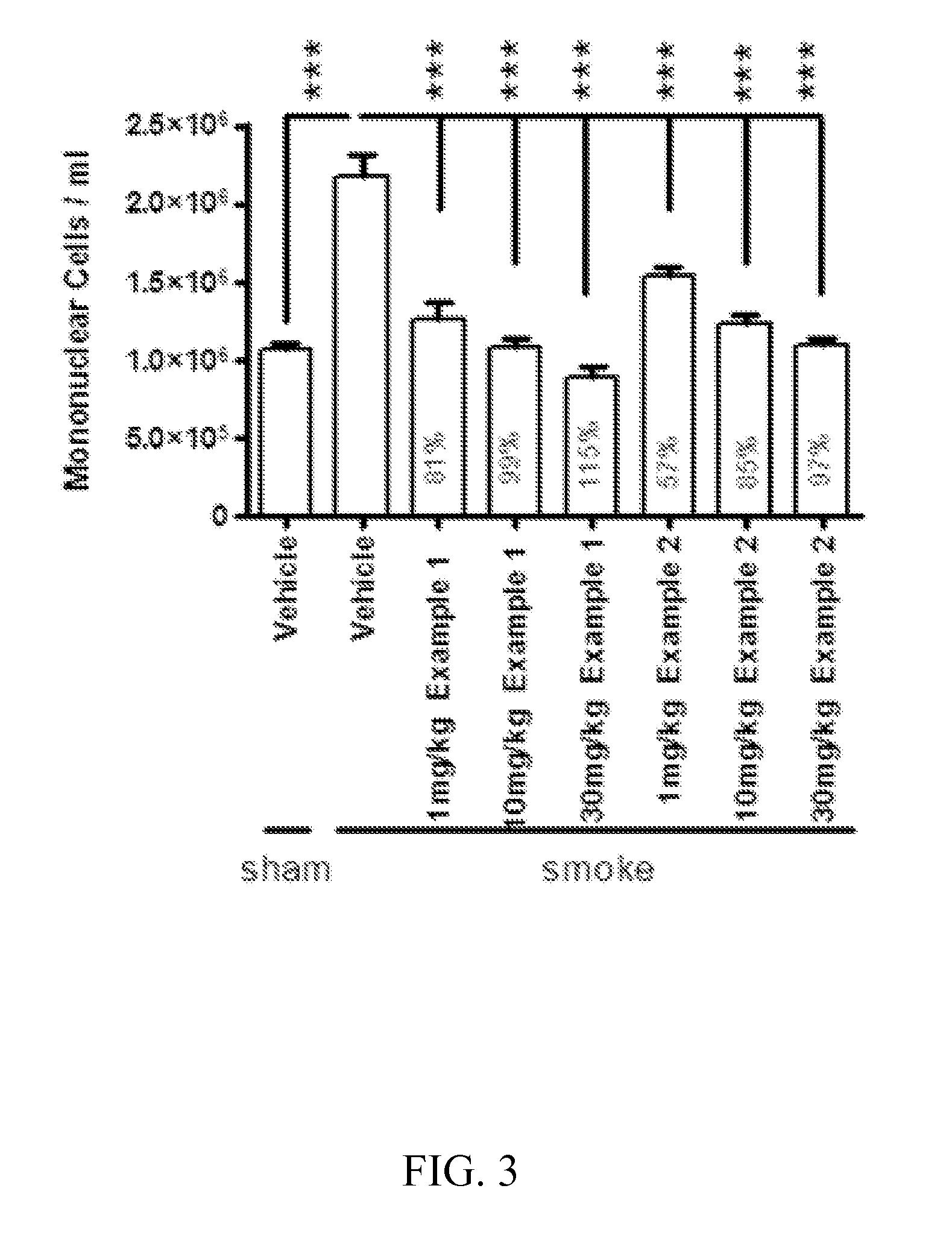
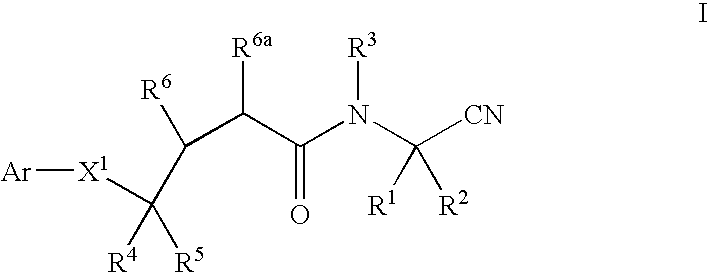
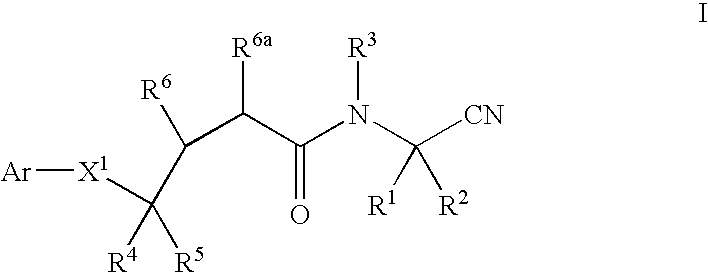
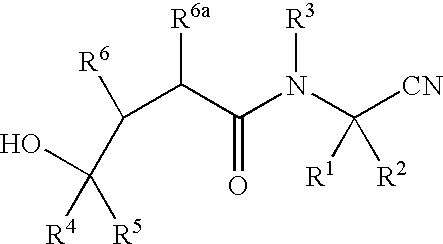
Methods for treating pulmonary emphysema using substituted 2-aza-bicyclo[2.2.1]heptane-3-carboxylic acid (benzyl-cyano-methyl)-amides inhibitors of cathepsin c
InactiveUS20150105375A1Potent Cathepsin C activityHigh selectivityAntibacterial agentsBiocideDiseaseCathepsin C
This invention relates to 2-Aza-bicyclo[2.2.1]heptane-3-carboxylic acid (benzyl-cyano-methyl)-amides of formula 1and their use as inhibitors of Cathepsin C, pharmaceutical compositions containing the same, and methods of using the same as agents for treatment and / or prevention of diseases connected with dipeptidyl peptidase I activity, e.g. respiratory diseases.
Owner:BOEHRINGER INGELHEIM INT GMBH
Substituted 2-aza-bicyclo[2.2.2]octane-3-carboxylic acid (benzyl-cyano-methyl)-amides inhibitors of cathepsin c
ActiveUS20140275159A1Potent Cathepsin C activityHigh selectivityAntibacterial agentsBiocideDiseaseDipeptidyl peptidase
This invention relates to 2-Aza-bicyclo[2.2.2]octane-3-carboxylic acid (benzyl-cyano-methyl)-amides of formula 1and their use as inhibitors of Cathepsin C, pharmaceutical compositions containing the same, and methods of using the same as agents for treatment and / or prevention of diseases connected with dipeptidyl peptidase I activity, e.g. respiratory diseases.
Owner:BOEHRINGER INGELHEIM INT GMBH
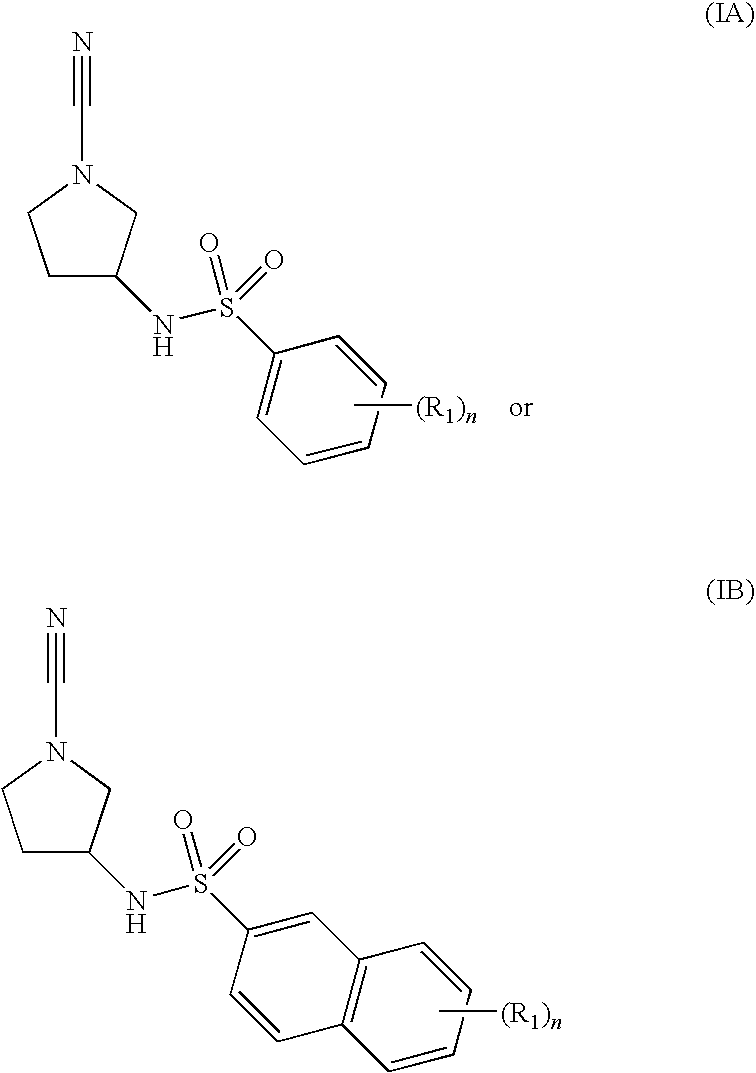
Cathepsin C Inhibitors
Owner:GLAXO GROUP LTD
Cathepsin cysteine protease inhibitors
This invention relates to a novel class of compounds which are cysteine protease inhibitors, including but not limited to, inhibitors of cathepsins K, L, S and B. These compounds are useful for treating diseases in which inhibition of bone resorption is indicated, such as osteoporosis.
Owner:MERCK CANADA INC
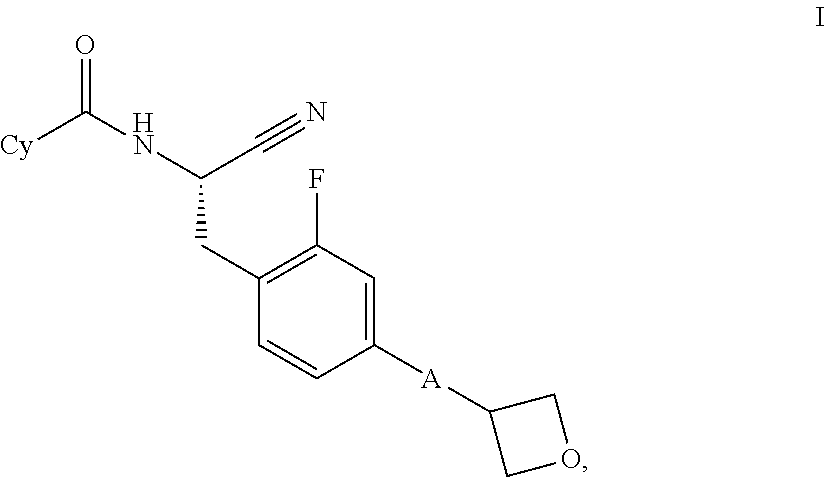
Substituted spirocycles
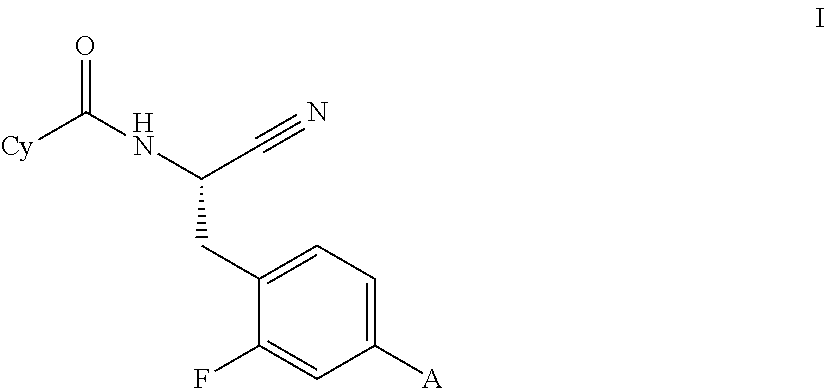
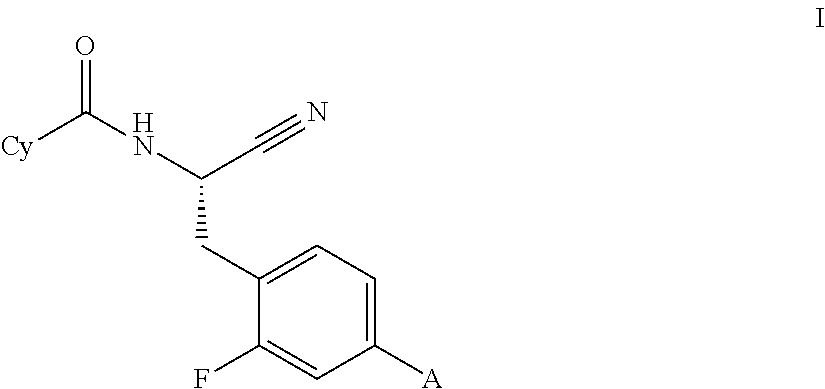
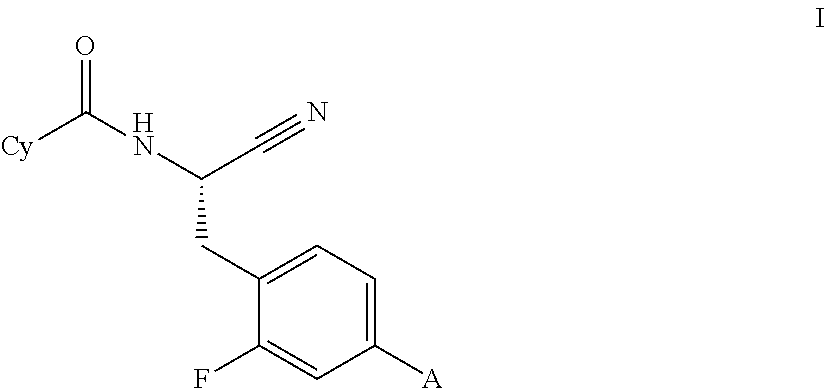
This invention relates to a compound of formula Iwherein A and Cy have one of the meanings as indicated in the specification and their use as inhibitors of Cathepsin C, pharmaceutical compositions containing the same and methods of using the same as agents for treatment and / or prevention of diseases connected with dipeptidyl peptidase I activity, e.g. respiratory diseases.
Owner:BOEHRINGER INGELHEIM INT GMBH
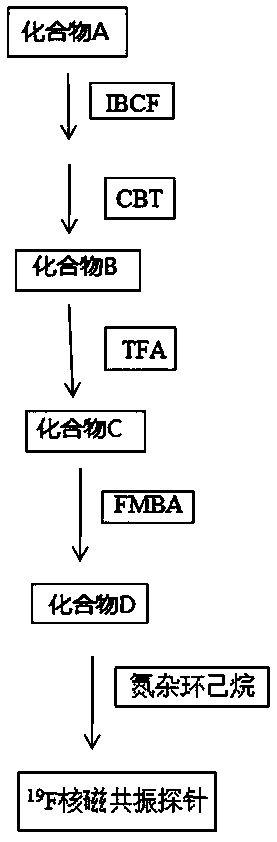
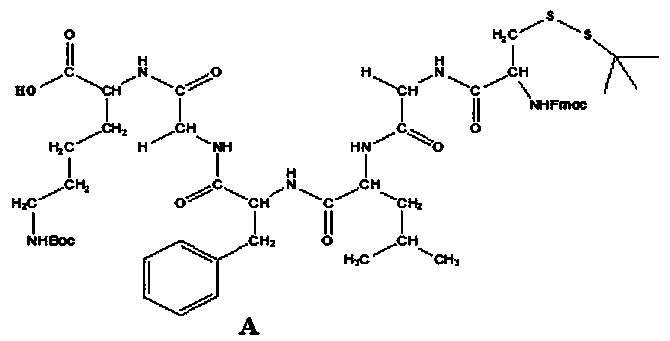
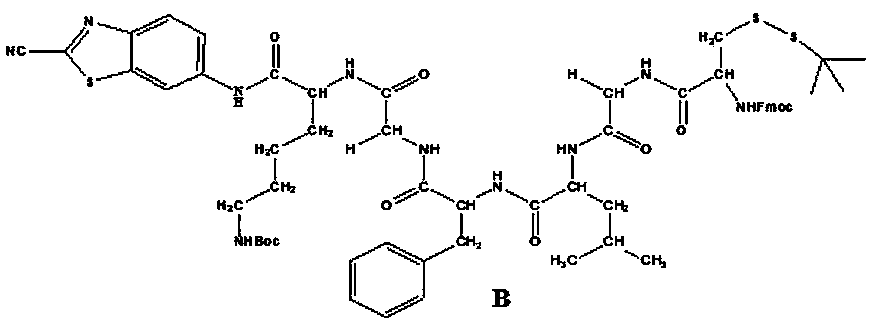
<19>F nuclear magnetic resonance probe, preparation method and applications thereof
InactiveCN108424764AHigh activityPeptide preparation methodsIn-vivo testing preparationsCathepsin KCathepsin O
The present invention provides a novel fluorine-containing <19>F nuclear magnetic resonance probe, which has a chemical formula of C49H58F6N10O7S3, has the following structural formula defined in thespecification, and can be used for detecting cathepsin B. According to the present invention, the magnetic resonance signal of the <19>F nuclear magnetic resonance probe is proportional to the activity of cathepsin B in vivo, the stronger the signal, the higher the activity of the cathepsin B, and the signal exceeding a certain threshold is related to malignant tumors, such that the new technicalway is provided for the early detection of tumors.
Owner:SOUTHERN MEDICAL UNIVERSITY
Cathepsin c inhibitors
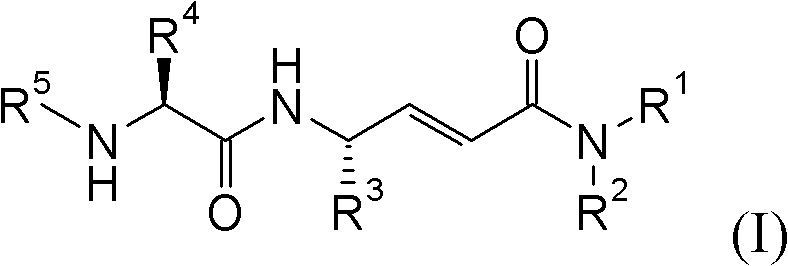
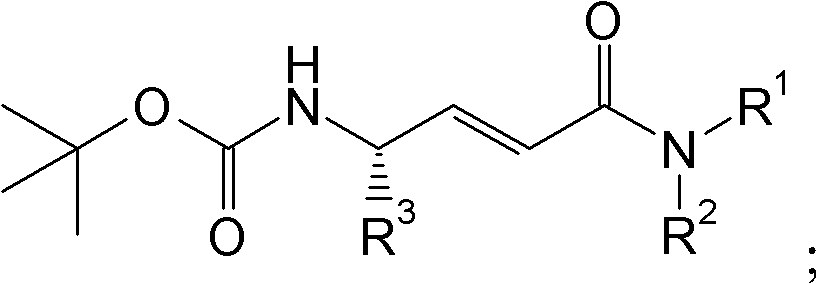
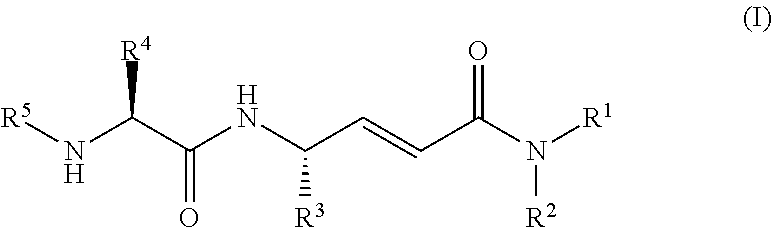
Disclosed are 4-amino-2-butenamides of Formula (I) having pharmacological activity, pharmaceutical compositions containing them, and methods for the treatment of diseases mediated by the cathepsin C enzyme such as chronic obstructive pulmonary disease.
Owner:GLAXO GROUP LTD
Cathepsin E as a marker of colon cancer
InactiveUS7892750B1Easy to implementNon-invasiveBiological material analysisPeptide preparation methodsDiseaseCathepsin K
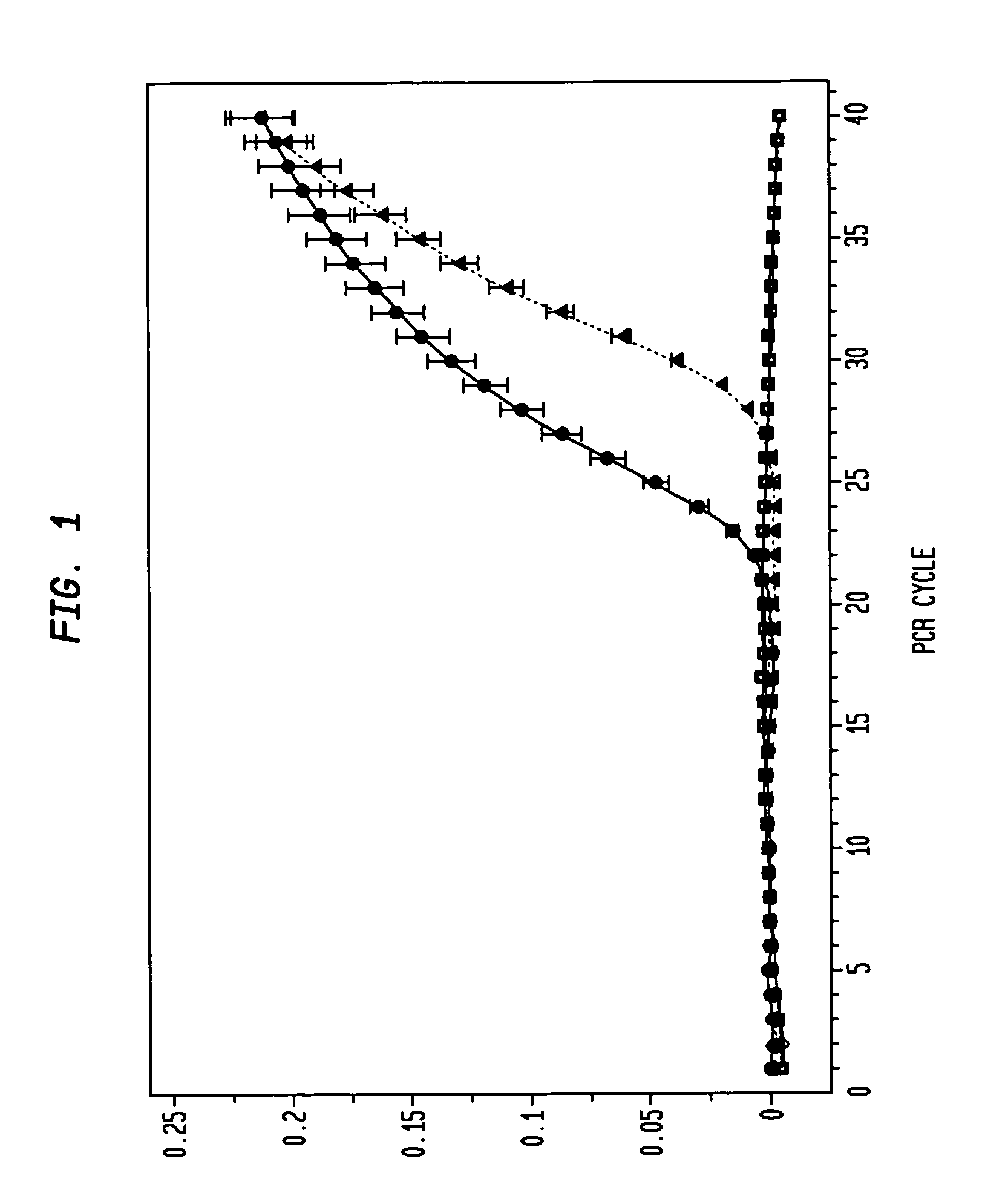
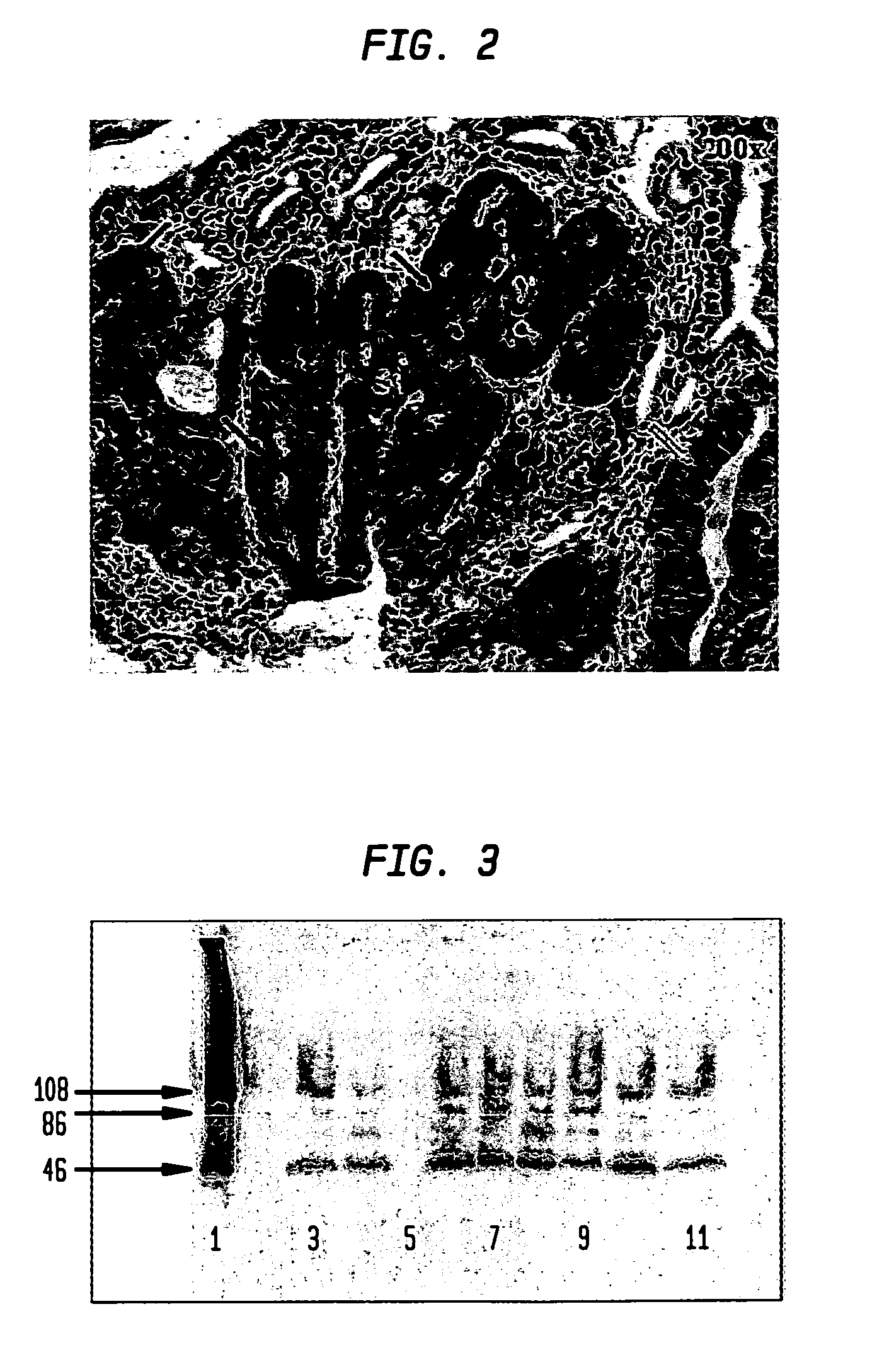
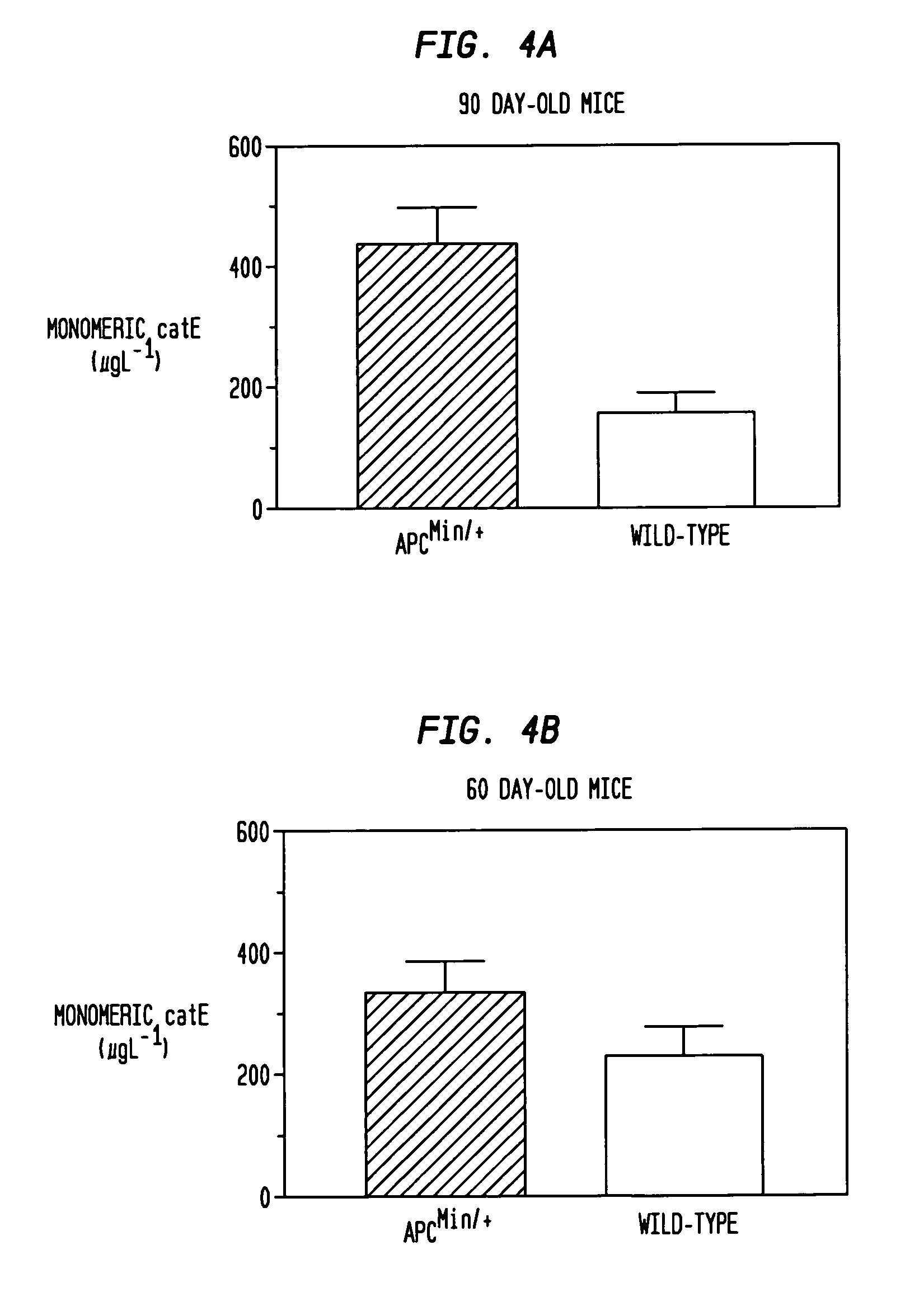
Elevated levels of cathepsin E (catE) are demonstrated to be diagnostic of intestinal forms of cancer, such as colorectal cancer. Elevated levels of cathepsin E (catE, monomeric forms) are demonstrated to be detectable in the urine of animals having colorectal cancer, and a diagnostic / screening method for identifying and / or detecting colorectal in an animal from a urine sample is provided. Specific tissue immunohistochemcial staining for catE (monomeric forms) in dysplastic tissue is also disclosed, and is shown to correlate with the level of dysplastic lesion severity. Hence, a method for determining and identifying dysplastic lesion severity is provided. Cathepsin E mRNA transcription and expression levels are also demonstrated to be upregulated in dysplastic tissue, relative to non-dysplastic tissue. Hence, a method for transcriptionally profiling an animal to monitor the progression of colorectal disease is provided.
Owner:UNIV OF NOTRE DAME DU LAC
Cathepsin L mediated diseases and associated methods and products
ActiveUS9144594B2Prevents actin disorganizationCompound screeningApoptosis detectionDiseaseCathepsin C
Owner:UNIV OF MIAMI +1
Novel substituted spirocycles
ActiveUS20160075704A1High inhibition of neutrophil elastase activityBiocideOrganic chemistryDiseaseDipeptidyl peptidase
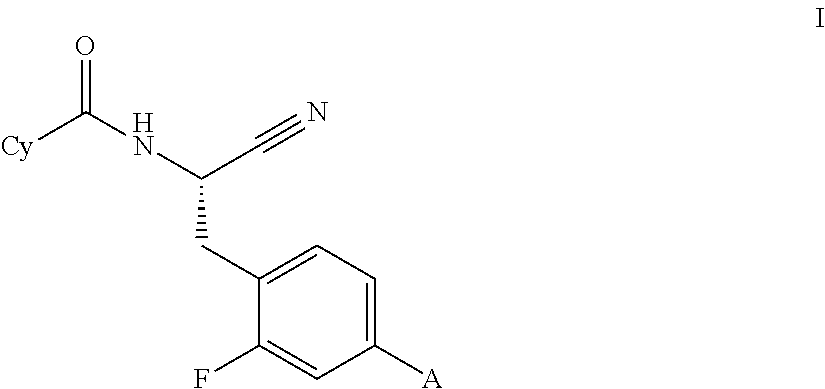
This invention relates to a compound of formula Iwherein A and Cy have one of the meanings as indicated in the specification and their use as inhibitors of Cathepsin C, pharmaceutical compositions containing the same and methods of using the same as agents for treatment and / or prevention of diseases connected with dipeptidyl peptidase I activity, e.g. respiratory diseases.
Owner:BOEHRINGER INGELHEIM INT GMBH
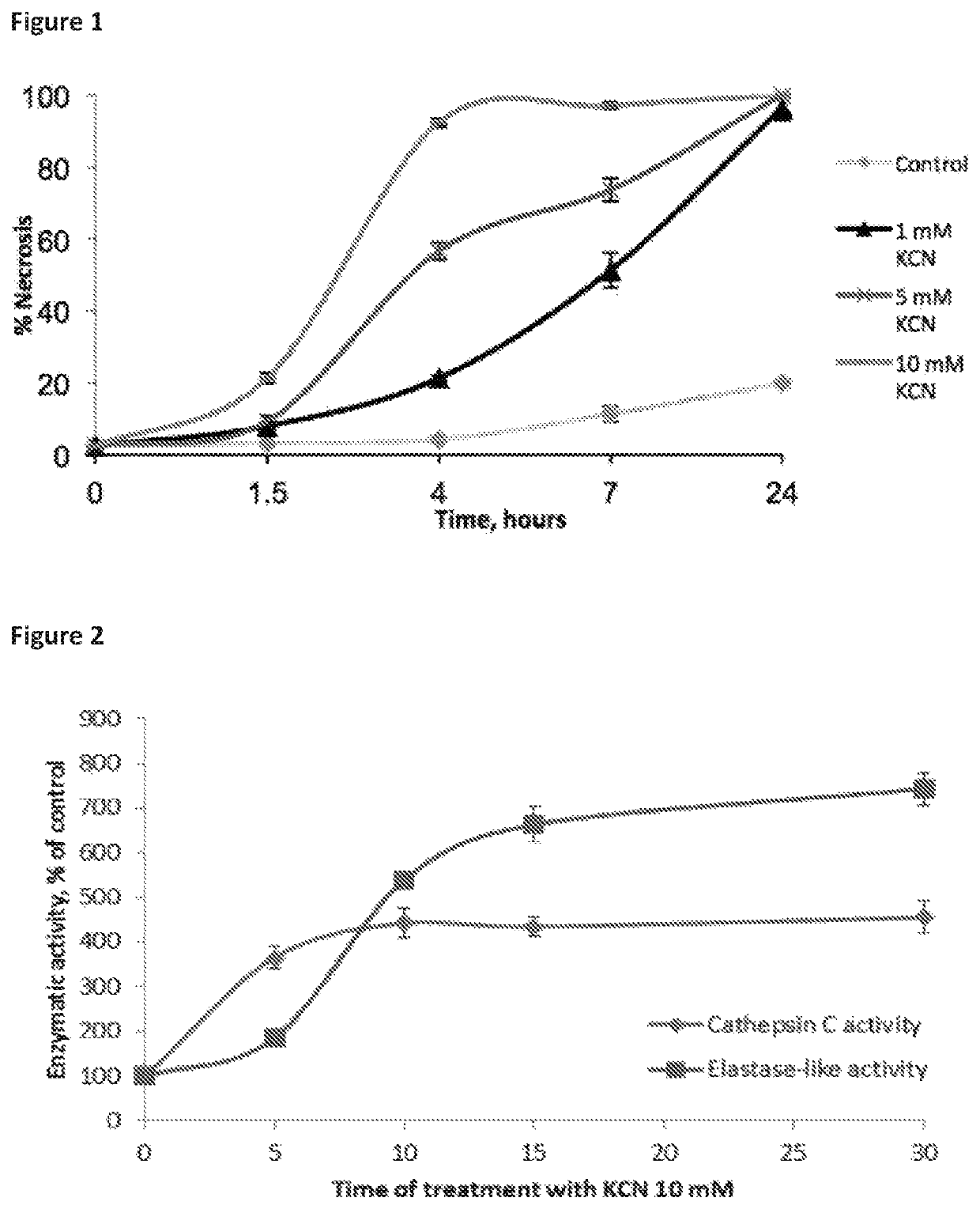
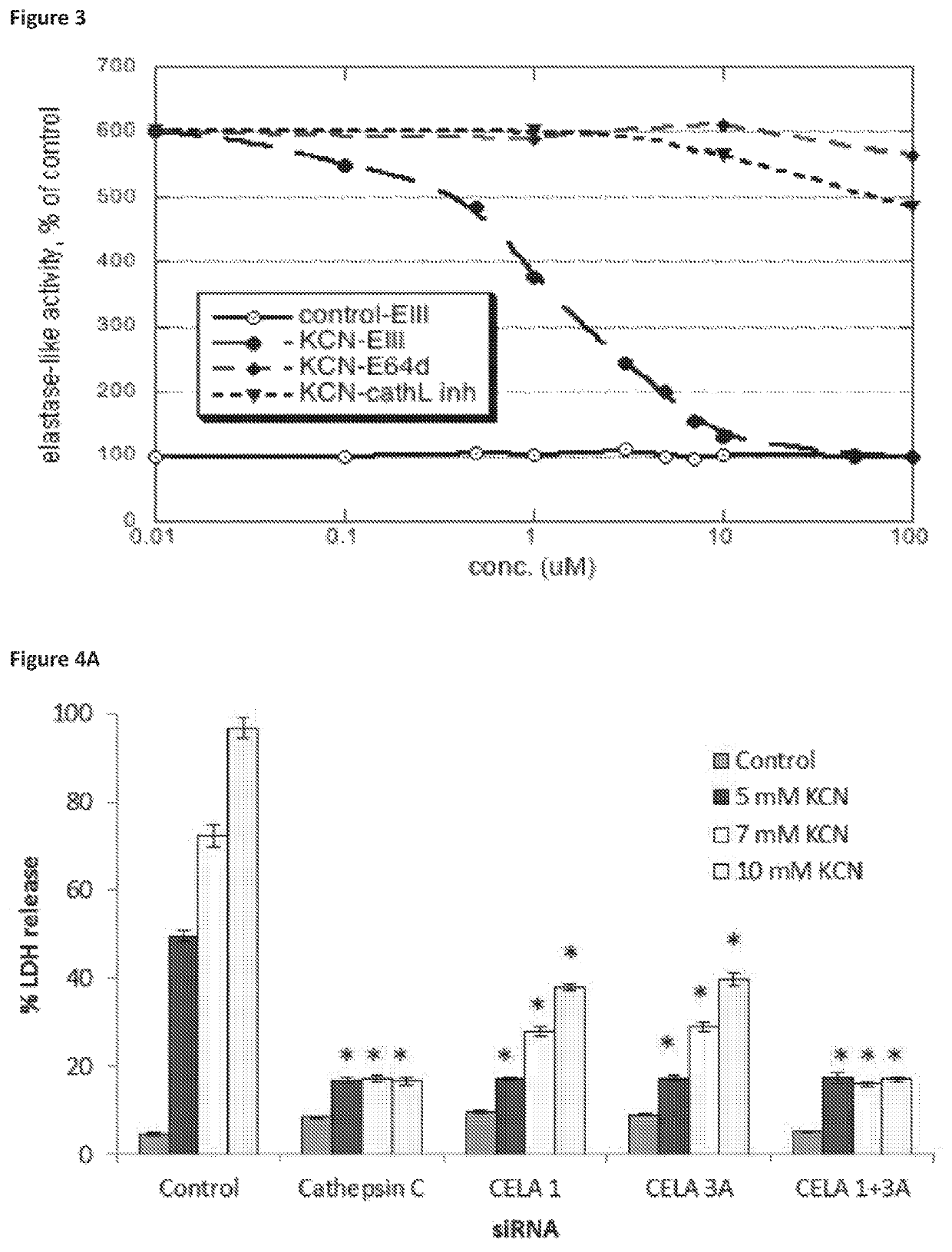
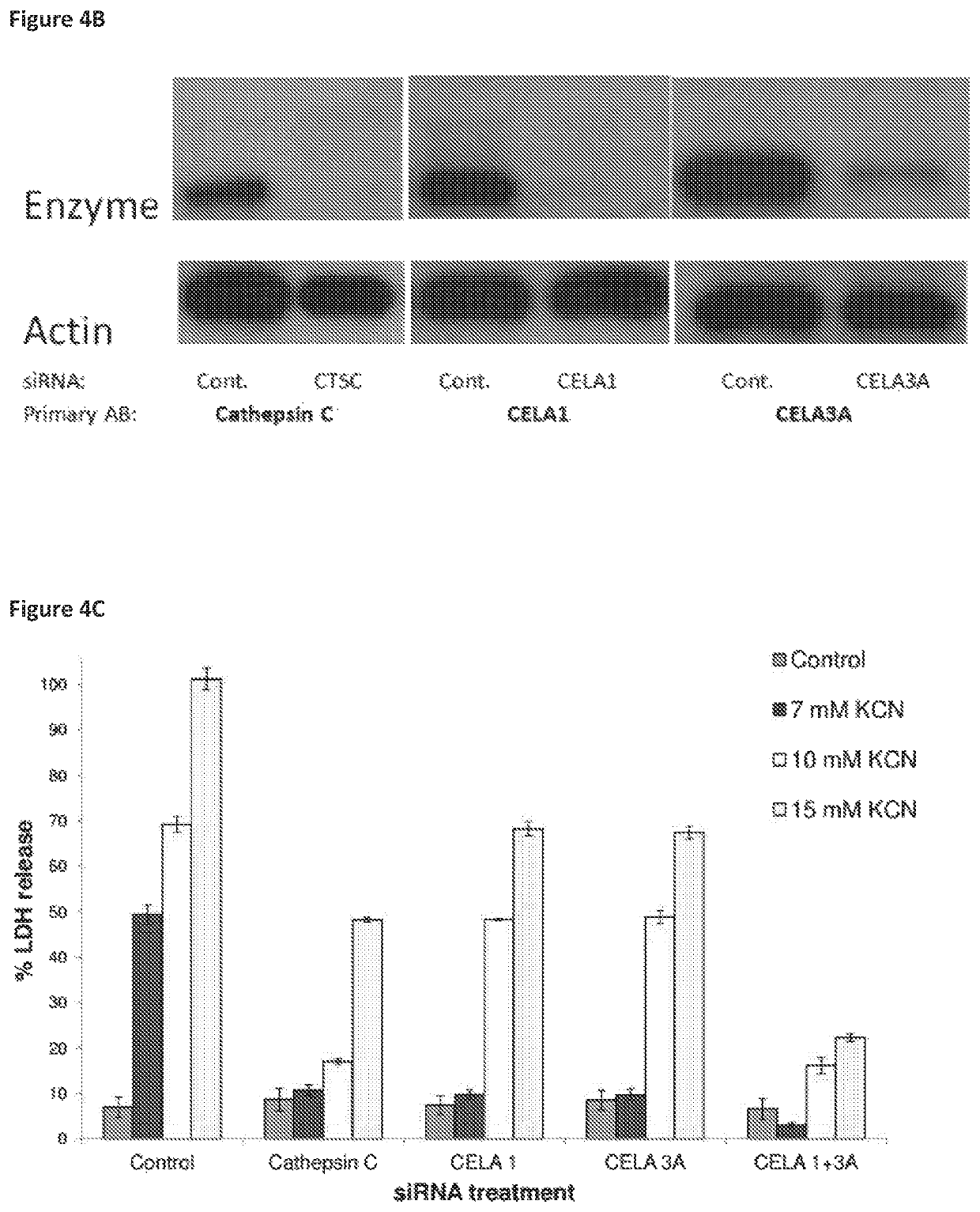
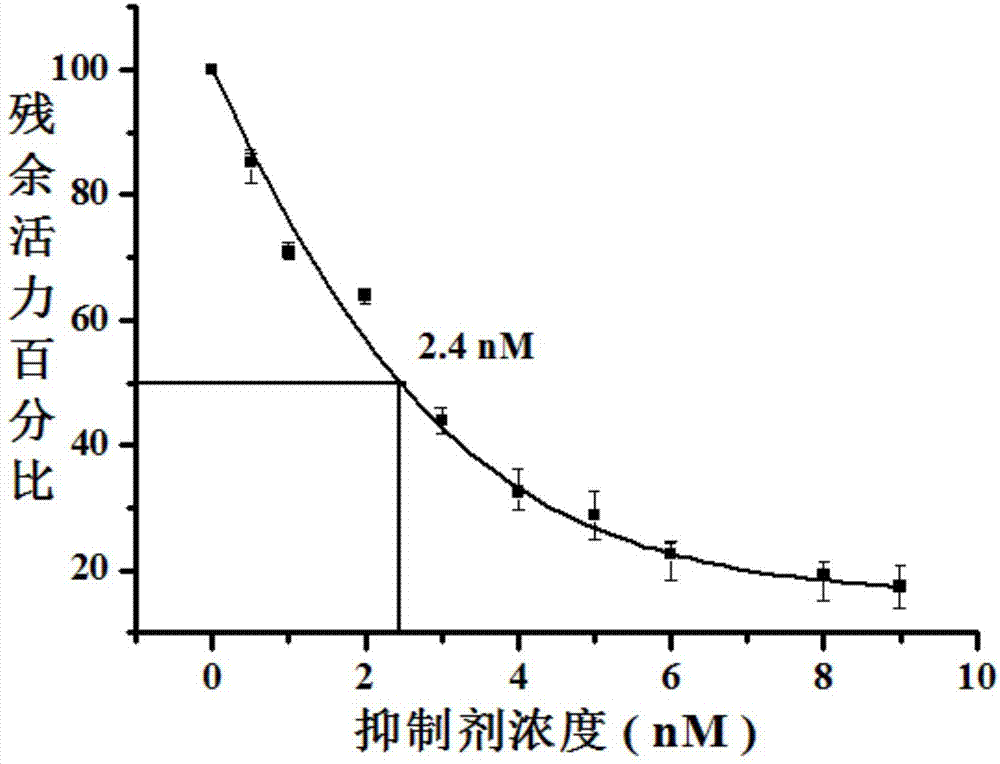
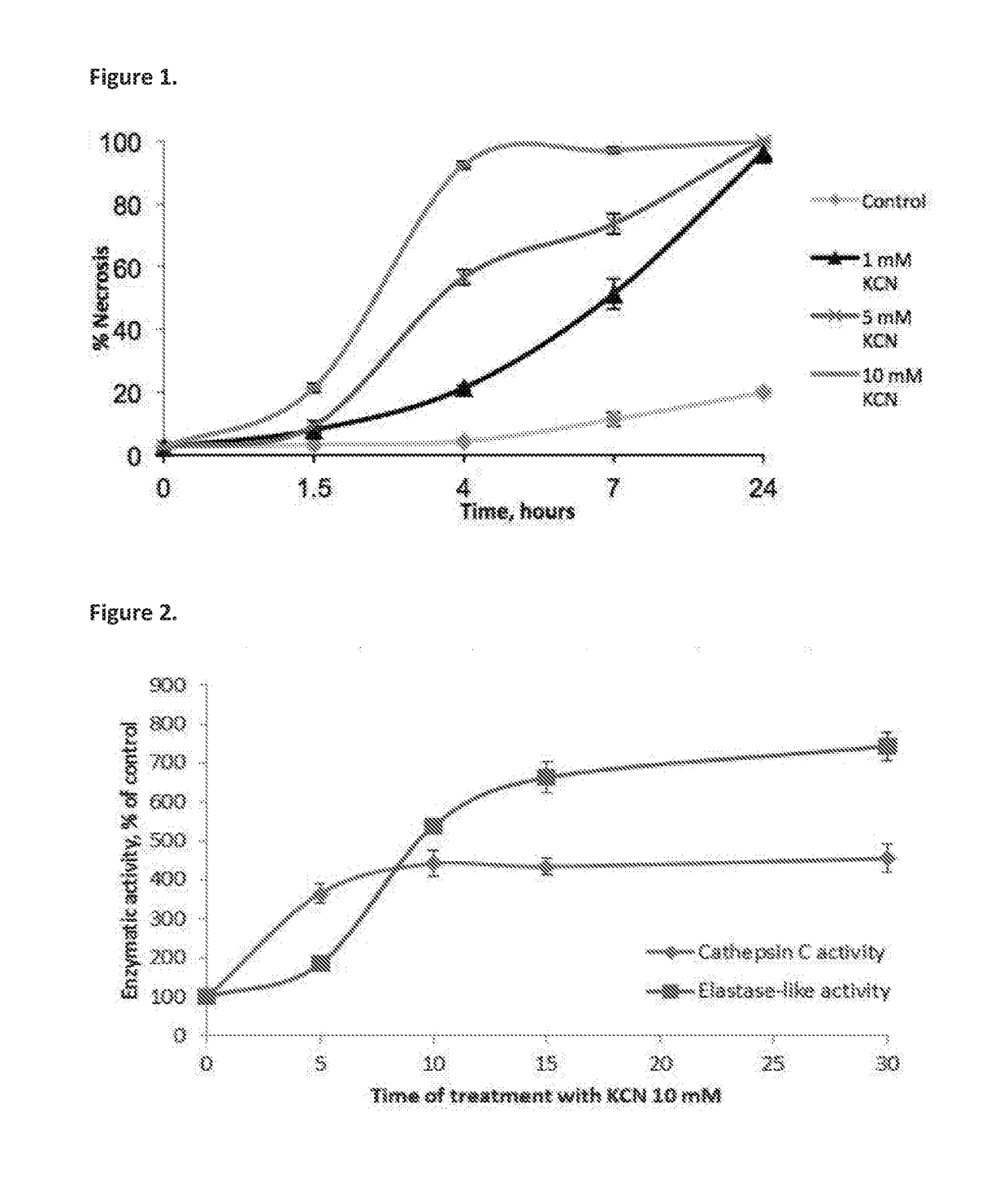
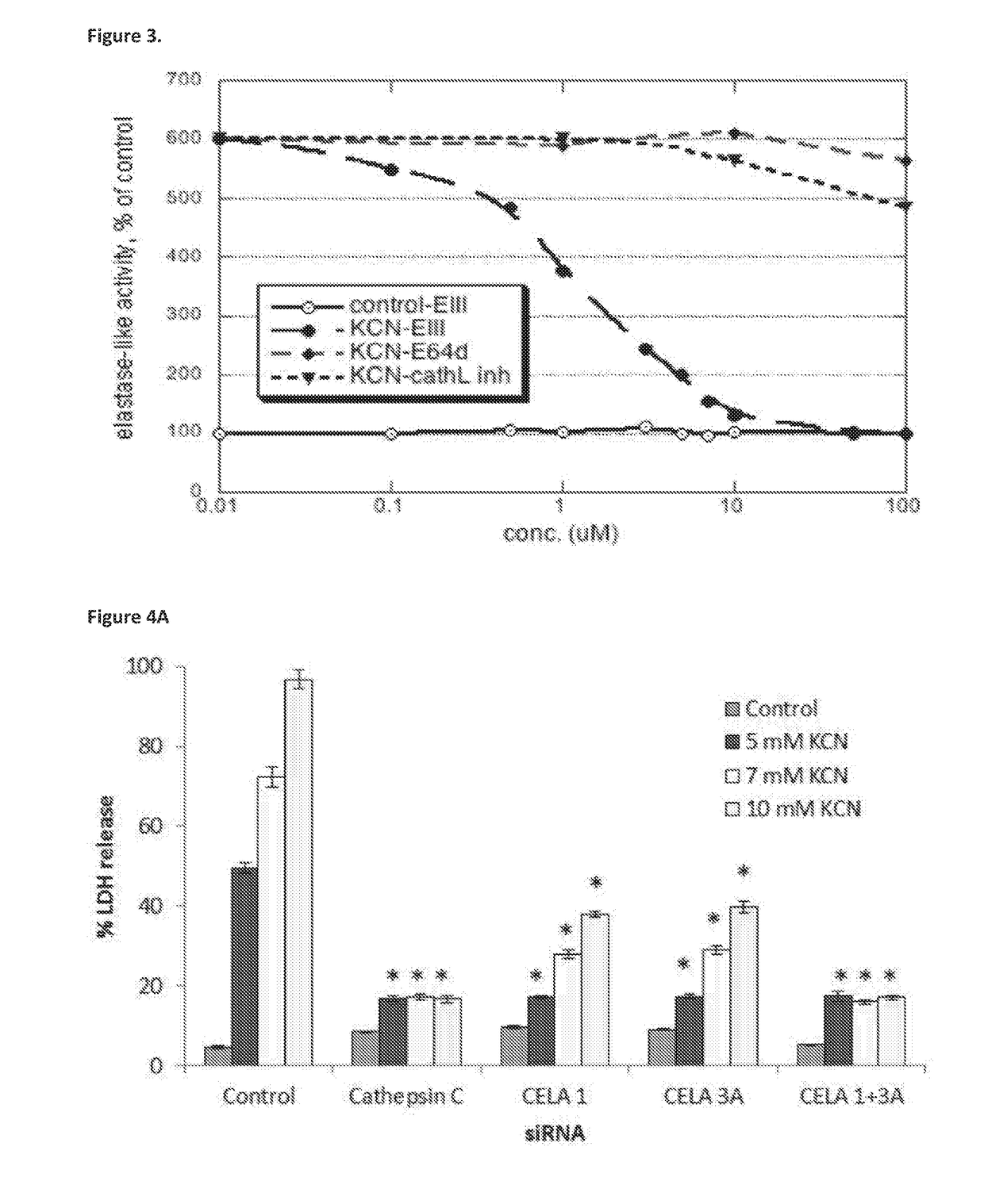
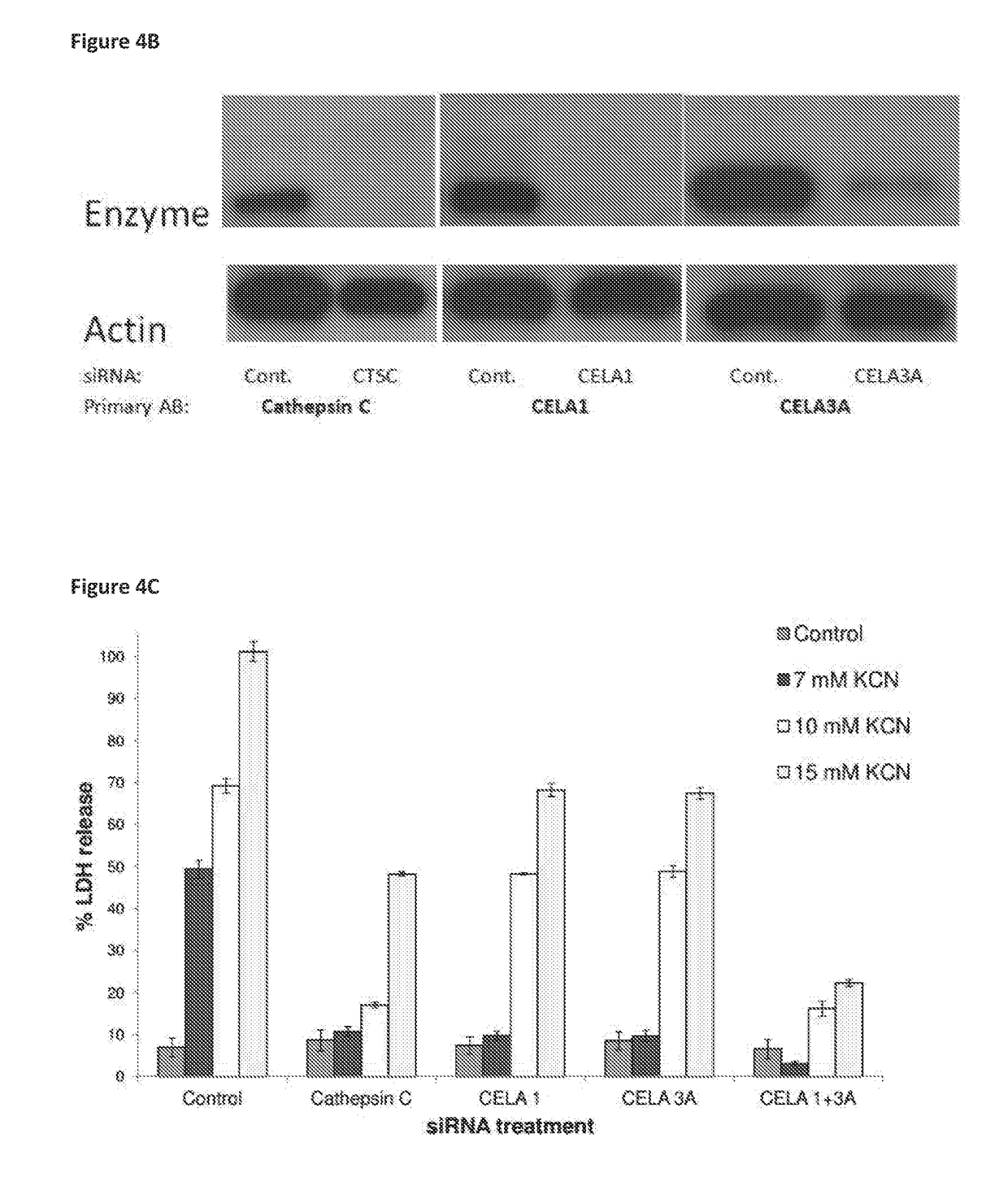
Compositions for treating and/or preventing cell or tissue necrosis specifically targeting cathepsin c and/or cela1 and/or cela3a and/or structurally related enzymes thereto
ActiveUS20180312477A1Highly effective preventionHighly effective treatmentAntibacterial agentsOrganic active ingredientsCathepsin CCombination therapy
Inhibitor compounds and agents of Cathepsin C, CELA1, CELA3A and / or structurally related molecules thereto, compositions comprising same and uses thereof in the inhibition and / or prevention of cell and / or tissue necrosis is described. Various applications for the described compounds, and combination therapies are described as well.
Owner:ELA PHARMA LTD
Cathepsin cysteine protease inhibitors
This invention relates to a novel class of compounds which are cysteine protease inhibitors, including but not limited to, inhibitors of cathepsins K, L, S and B. These compounds are useful for treating diseases in which inhibition of bone resorption is indicated, such as osteoporosis.
Owner:MERCK SHARP & DOHME LLC
Cyanoalkylamino derivatives as protease inhibitors
The present invention is directed to novel cyanoalkylamino derivatives that are inhibitors of cysteine protease such as cathepsins K, S, B and L, in particular cathepsin K Pharmaceutical composition comprising these compounds, method of treating diseases mediated by unregulated cysteine protease activity, in particular cathepsin K utilizing these compounds and methods of preparing these compounds are also disclosed.
Owner:AXYX PHARMA INC +1
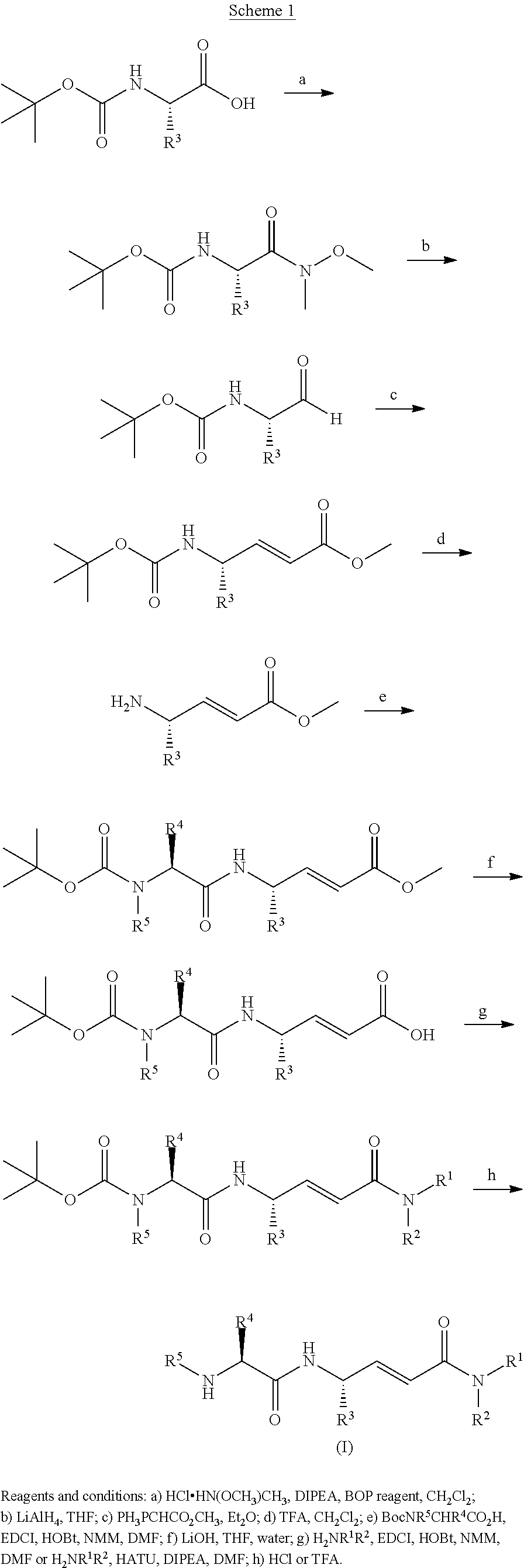
Methods for treating pulmonary emphysema using substituted 2-aza-bicyclo[2.2.1]heptane-3-carboxylic acid (benzyl-cyano-methyl)-amides inhibitors of cathepsin c
ActiveUS20160081982A1Potent Cathepsin C activityHigh selectivityAntibacterial agentsOrganic active ingredientsDiseaseCathepsin C
This invention relates to 2-Aza-bicyclo[2.2.1]heptane-3-carboxylic acid (benzyl-cyano-methyl)-amides of formula 1and their use as inhibitors of Cathepsin C, pharmaceutical compositions containing the same, and methods of using the same as agents for treatment and / or prevention of diseases connected with dipeptidyl peptidase I activity, e.g. respiratory diseases.
Owner:BOEHRINGER INGELHEIM INT GMBH
Substituted thiazoles for preventing and/or treating cell or tissue necrosis
ActiveUS10968185B2Preventing and treating and halting/abrogating necrosisGood lookingAntibacterial agentsOrganic active ingredientsCathepsin CCombination therapy
Inhibitor compounds and agents of Cathepsin C, CELA1, CELA3A and / or structurally related molecules thereto, compositions comprising same and uses thereof in the inhibition and / or prevention of cell and / or tissue necrosis are described. The compounds include thiazoles of Formula II,where G1 is an optionally substituted pyrrolidine, an optionally substituted pyridine, an optionally substituted aryl, an optionally substituted piperidine, an optionally substituted piperazine, an optionally substituted imidazolidine, or an optionally substituted pyrazolidine. G3 isor G3 is an optionally substituted alkyl, an optionally substituted aryl or an optionally cyloalkyl. G2 is an optionally substituted alkyl, an optionally substituted aryl, an optionally substituted cyloalkyl or an optionally substituted heterocycle. Various applications for the described compounds, and combination therapies are described as well.
Owner:ELA PHARMA LTD
Hydrazine nitrile cathepsin K inhibitor and its application in the preparation of medicines for treating osteoarthritis
InactiveCN105837479BEnhanced inhibitory effectReduced activityOrganic chemistryAntipyreticCathepsin KCathepsin O
The invention relates to a hydrazinonitrile cathepsin K inhibitor, and an application thereof in preparation of osteoarthritis treatment drugs, and belongs to the technical field of cathepsin inhibitors. The inhibitor contains a novel P3 group with different orientations, has a nano-mole concentration level inhibition effect on cathepsin K outside cells, has hundreds-of-times or above selectivity to cathepsin K, cathepsin S and cathepsin B, and also has hundreds-of-times or above selectivity to highly homologous cathepsin K and cathepsin L. Culture is carried out under the following two conditions: (1) cell passage and (2) ascorbic acid and beta-glycerophosphoric acid stimulation with primary chondrocytes as a model. Results of gelatin zymography and quantitative fluorescence assay tests show that the expression level of the cathepsin K under every one of the above two conditions is increased; and the activity of the cathepsin K is reduced after the novel inhibitor is added. The inhibitor also has a good inhibition effect in the cells, and has a very good inhibition effect on the cathepsin K.
Owner:JILIN UNIV
Cathepsin C inhibitors
Disclosed are 4-amino-2-butenamides of Formula (I) having pharmacological activity, pharmaceutical compositions containing them, and methods for the treatment of diseases mediated by the cathepsin C enzyme such as chronic obstructive pulmonary disease.
Owner:GLAXOSMITHKLINE INTPROP DEV LTD
Cathepsin C inhibitors
PendingUS20210114983A1Maintain cathepsin C inhibitory activityOrganic chemistryImmunological disordersCathepsin CMedicine
Disclosed compounds, pharmaceutical compositions are used for inhibiting cathepsin C without inhibiting epidermal growth factor receptor (EGFR).
Owner:NAT INST OF BIOLOGICAL SCI BEIJING
Application of CTSG (cathepsin G) cell factor in preparation of medicaments to treat hepatic failure
InactiveCN110201149ASuppress deathInhibit apoptosisPeptide/protein ingredientsDigestive systemUpper gastrointestinalCathepsin G
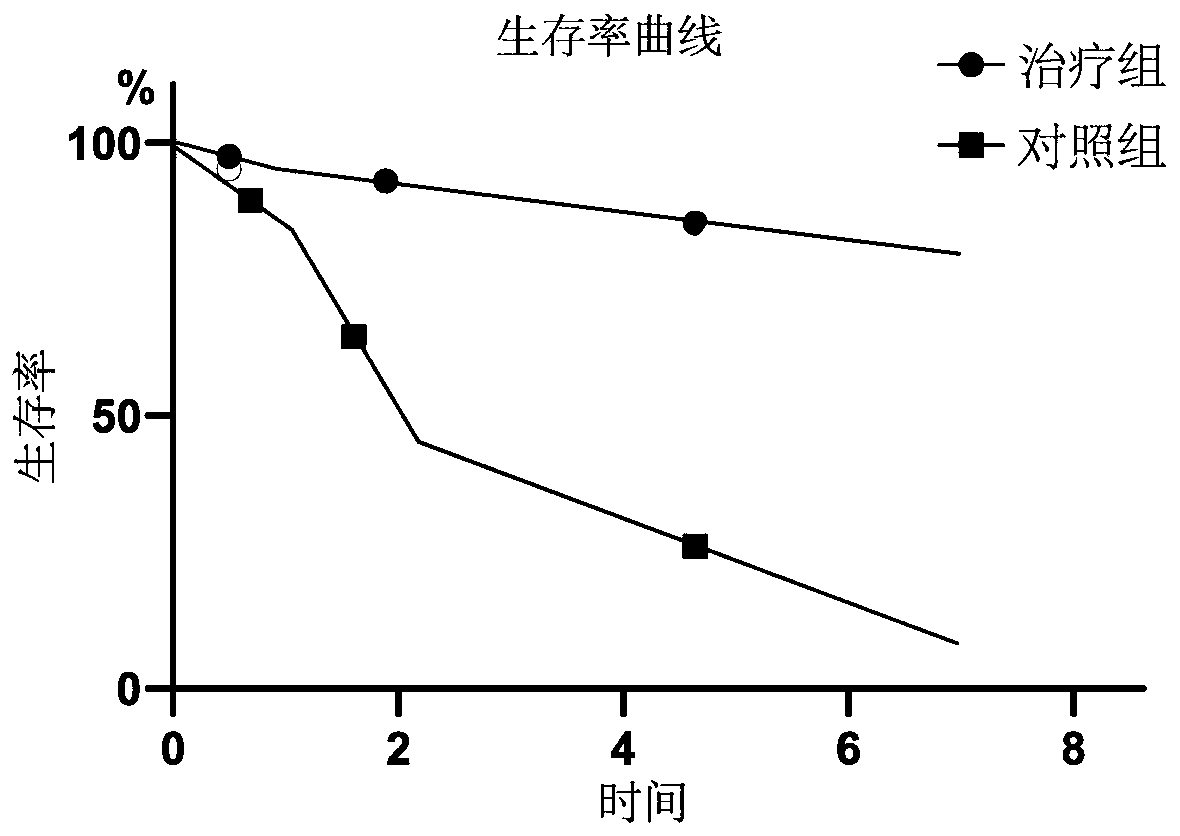
The invention discloses application of a CTSG (cathepsin G) cell factor in the preparation of medicaments to treat hepatic failure. Death of mass hepatic cells occurs during the course of hepatic failure; the CTSG cell factor helps overcome the aforementioned problem. CTSG JNK (c-Jun N-terminal kinase) is one of MAPK (mitogen-activated protein kinase) family members; JNK pathway can inhibit apoptosis of hepatic cells by regulating the expression of CTSG. The CTSG cell factor can be applied to the preparation of medicaments to treat hepatic failure to significantly increase biochemical indexesof a patient, reduce bilirubin level, lower aminotransferase, improve the coagulation function, inhibit cell apoptosis, promote the regeneration of hepatic cells and bile duct cells, prevent occurrence of fatal complications, such as massive alimentary tract bleeding, severe hepatic encephalopathy and hepatorenal syndrome, extend the survival time of the patient significantly, and increase the survival rate.
Owner:杭州笙源生物科技有限公司
Cathepsin cysteine protease inhibitors
This invention relates to a novel class of compounds which are cysteine protease inhibitors, including but not limited to, inhibitors of cathepsins K, L, S and B. These compounds are useful for treating diseases in which inhibition of bone resorption is indicated, such as osteoporosis.
Owner:MERCK SHARP & DOHME LLC
Therapy targeting cathepsin S
InactiveUS9321838B2Enhanced cell surface localisationImprove the degradation problemOrganic active ingredientsSenses disorderCathepsin CCathepsin K
Owner:FUSION ANTIBODIES
Cathepsin C inhibitors
Disclosed are 4-amino-2-butenamides of Formula (I) having pharmacological activity, pharmaceutical compositions containing them, and methods for the treatment of diseases mediated by the cathepsin C enzyme such as chronic obstructive pulmonary disease.
Owner:GLAXO GRP LTD
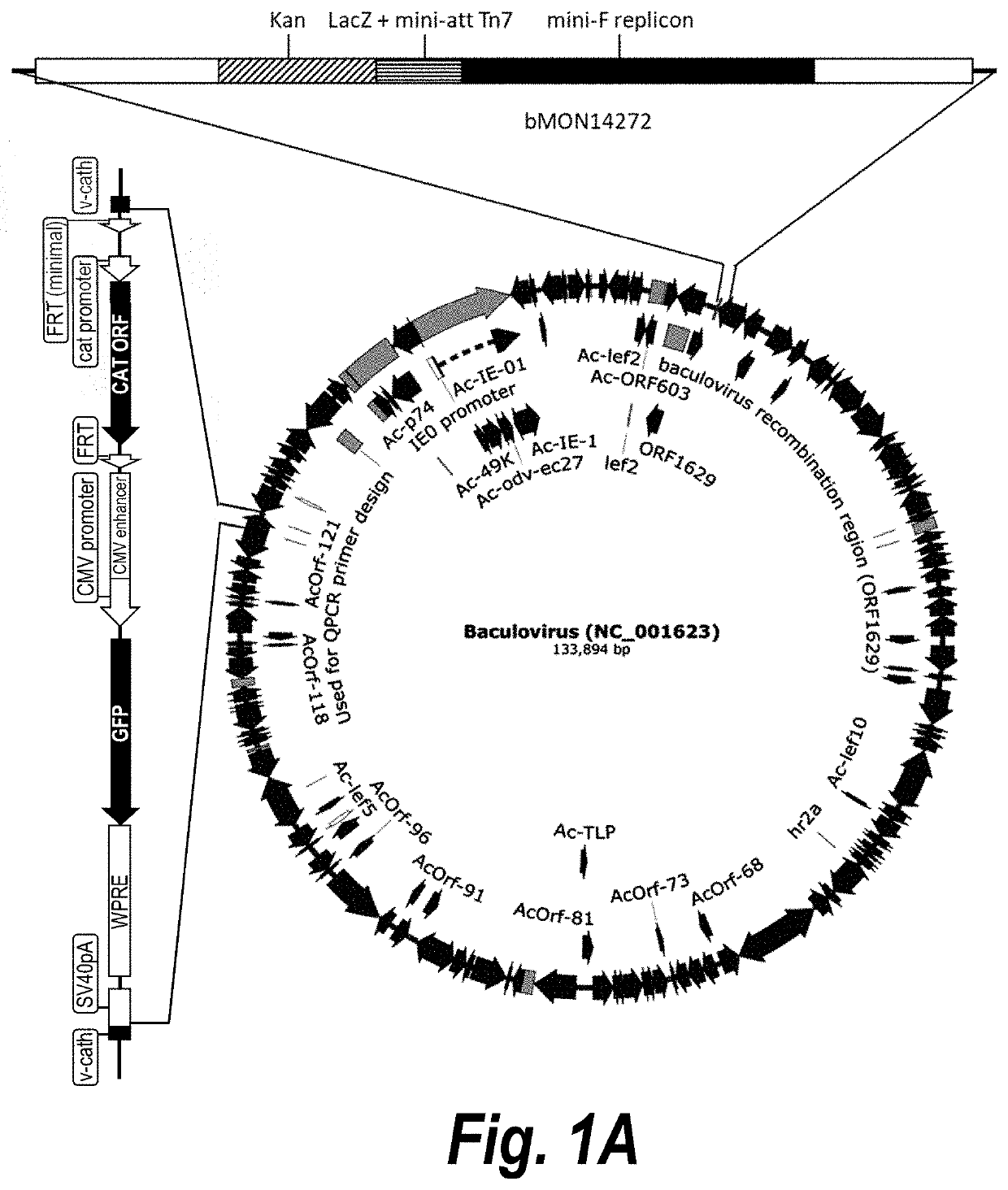
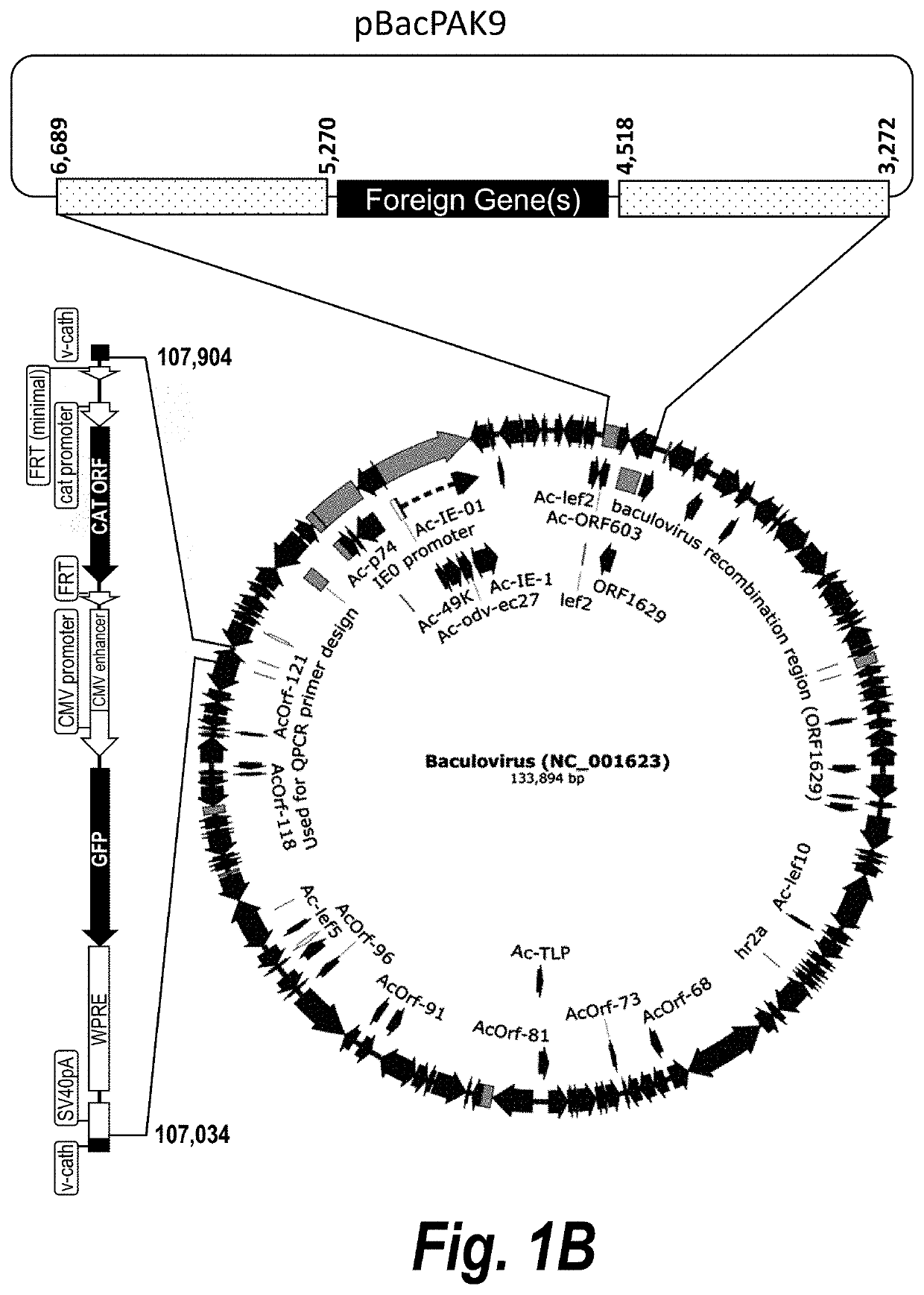
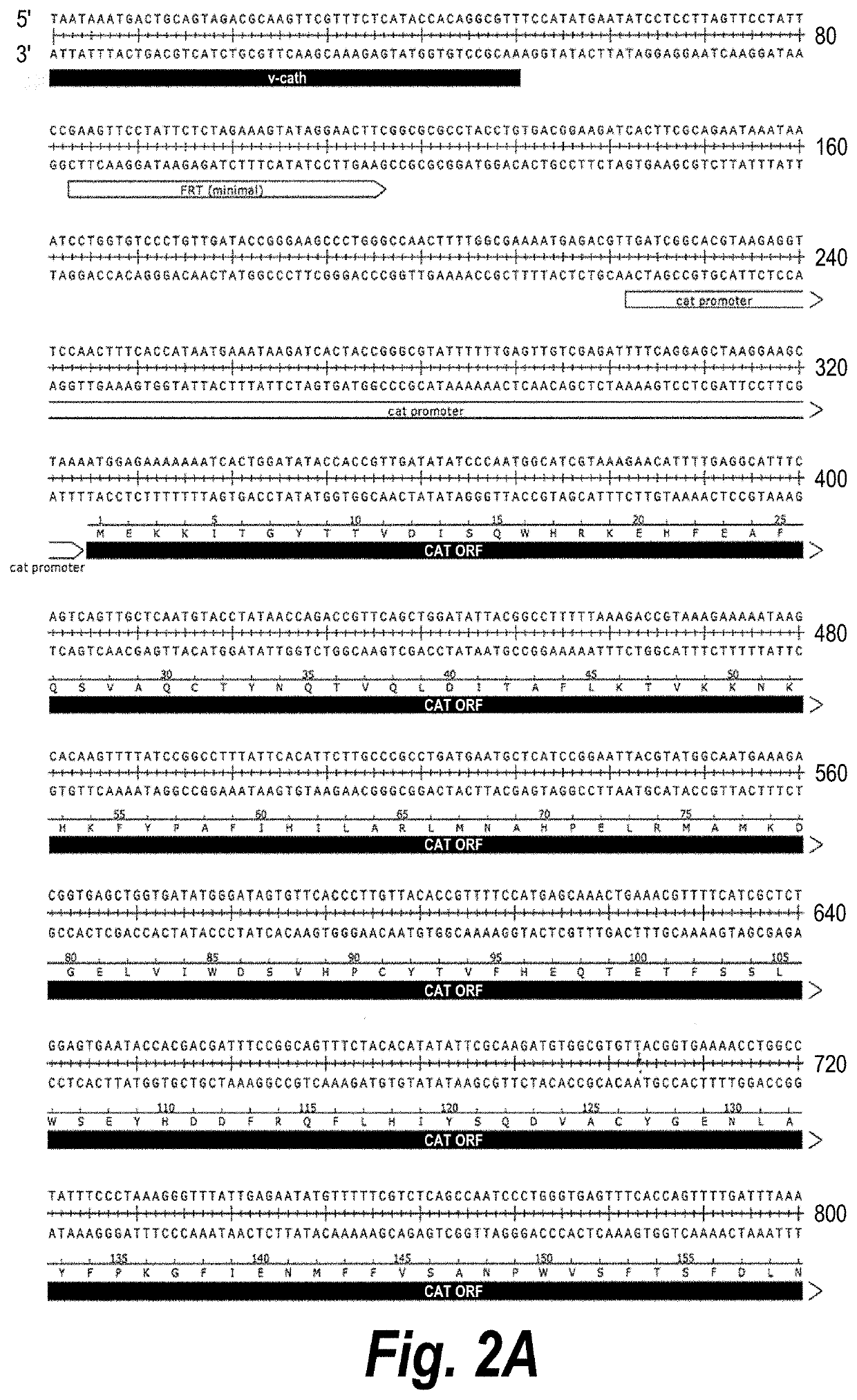
Baculovirus expression system
PendingUS20200407696A1Improves Structural IntegrityIncrease infectivityMicroorganismsVirus peptidesHeterologousCathepsin C
The present disclosure relates to a heterologous recombinant baculovirus (rBV) expression system for the production of foreign heterologous proteins in insect cells. This system comprises a recombinant baculovirus backbone within a genome with a deletion in the cathepsin gene into which foreign gene cassettes can be integrated, and an insect cell that can be infected by the Δv-cath-rBV, and in which the foreign proteins and / or viral vectors or particles are expressed.
Owner:VIROVEK
Substituted oxetanes and their use as inhibitors of cathepsin c
This invention relates to a compound of formula Iand their use as inhibitors of Cathepsin C, pharmaceutical compositions containing the same, and methods of using the same as agents for treatment and / or prevention of diseases connected with dipeptidyl peptidase I activity, e.g. respiratory diseases.
Owner:BOEHRINGER INGELHEIM INT GMBH
Cathepsin c inhibitors
Disclosed are 4-amino-2-butenamides of Formula (I) having pharmacological activity, pharmaceutical compositions containing them, and methods for the treatment of diseases mediated by the cathepsin C enzyme such as chronic obstructive pulmonary disease.
Owner:GLAXOSMITHKLINE INTPROP DEV LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Substituted N- [1-cyano-2- (phenyl) ethyl] -2-azabicyclo [2.2.1] heptane-3-carboxamide inhibitors of cathepsin C Substituted N- [1-cyano-2- (phenyl) ethyl] -2-azabicyclo [2.2.1] heptane-3-carboxamide inhibitors of cathepsin C](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/60a8808d-cb20-468b-856e-16ac212fdf4e/US08999975-20150407-C00001.PNG)
![Substituted N- [1-cyano-2- (phenyl) ethyl] -2-azabicyclo [2.2.1] heptane-3-carboxamide inhibitors of cathepsin C Substituted N- [1-cyano-2- (phenyl) ethyl] -2-azabicyclo [2.2.1] heptane-3-carboxamide inhibitors of cathepsin C](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/60a8808d-cb20-468b-856e-16ac212fdf4e/US08999975-20150407-C00002.PNG)
![Substituted N- [1-cyano-2- (phenyl) ethyl] -2-azabicyclo [2.2.1] heptane-3-carboxamide inhibitors of cathepsin C Substituted N- [1-cyano-2- (phenyl) ethyl] -2-azabicyclo [2.2.1] heptane-3-carboxamide inhibitors of cathepsin C](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/60a8808d-cb20-468b-856e-16ac212fdf4e/US08999975-20150407-C00003.PNG)
![Substituted 2-aza-bicyclo[2.2.2]octane-3-carboxylic acid (benzyl-cyano-methyl)-amides inhibitors of cathepsin C Substituted 2-aza-bicyclo[2.2.2]octane-3-carboxylic acid (benzyl-cyano-methyl)-amides inhibitors of cathepsin C](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/83abf9fb-fcd1-4680-ae03-baf73703c1ac/US08877775-20141104-C00001.png)
![Substituted 2-aza-bicyclo[2.2.2]octane-3-carboxylic acid (benzyl-cyano-methyl)-amides inhibitors of cathepsin C Substituted 2-aza-bicyclo[2.2.2]octane-3-carboxylic acid (benzyl-cyano-methyl)-amides inhibitors of cathepsin C](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/83abf9fb-fcd1-4680-ae03-baf73703c1ac/US08877775-20141104-C00002.png)
![Substituted 2-aza-bicyclo[2.2.2]octane-3-carboxylic acid (benzyl-cyano-methyl)-amides inhibitors of cathepsin C Substituted 2-aza-bicyclo[2.2.2]octane-3-carboxylic acid (benzyl-cyano-methyl)-amides inhibitors of cathepsin C](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/83abf9fb-fcd1-4680-ae03-baf73703c1ac/US08877775-20141104-C00003.png)


![Substituted 2-aza-bicyclo[2.2.1]heptane-3-carboxylic acid (cyano-methyl)-amides inhibitors of cathepsin C Substituted 2-aza-bicyclo[2.2.1]heptane-3-carboxylic acid (cyano-methyl)-amides inhibitors of cathepsin C](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ebaea0a0-7945-4f40-9104-ec3abef3b560/US08871783-20141028-C00001.png)
![Substituted 2-aza-bicyclo[2.2.1]heptane-3-carboxylic acid (cyano-methyl)-amides inhibitors of cathepsin C Substituted 2-aza-bicyclo[2.2.1]heptane-3-carboxylic acid (cyano-methyl)-amides inhibitors of cathepsin C](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ebaea0a0-7945-4f40-9104-ec3abef3b560/US08871783-20141028-C00002.png)
![Substituted 2-aza-bicyclo[2.2.1]heptane-3-carboxylic acid (cyano-methyl)-amides inhibitors of cathepsin C Substituted 2-aza-bicyclo[2.2.1]heptane-3-carboxylic acid (cyano-methyl)-amides inhibitors of cathepsin C](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ebaea0a0-7945-4f40-9104-ec3abef3b560/US08871783-20141028-C00003.png)
![Methods for treating pulmonary emphysema using substituted 2-aza-bicyclo[2.2.1]heptane-3-carboxylic acid (benzyl-cyano-methyl)-amides inhibitors of cathepsin c Methods for treating pulmonary emphysema using substituted 2-aza-bicyclo[2.2.1]heptane-3-carboxylic acid (benzyl-cyano-methyl)-amides inhibitors of cathepsin c](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/7fc62292-a1ac-4419-9baf-dbf2a9b1f447/US20150105375A1-20150416-C00001.PNG)
![Methods for treating pulmonary emphysema using substituted 2-aza-bicyclo[2.2.1]heptane-3-carboxylic acid (benzyl-cyano-methyl)-amides inhibitors of cathepsin c Methods for treating pulmonary emphysema using substituted 2-aza-bicyclo[2.2.1]heptane-3-carboxylic acid (benzyl-cyano-methyl)-amides inhibitors of cathepsin c](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/7fc62292-a1ac-4419-9baf-dbf2a9b1f447/US20150105375A1-20150416-C00002.PNG)
![Methods for treating pulmonary emphysema using substituted 2-aza-bicyclo[2.2.1]heptane-3-carboxylic acid (benzyl-cyano-methyl)-amides inhibitors of cathepsin c Methods for treating pulmonary emphysema using substituted 2-aza-bicyclo[2.2.1]heptane-3-carboxylic acid (benzyl-cyano-methyl)-amides inhibitors of cathepsin c](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/7fc62292-a1ac-4419-9baf-dbf2a9b1f447/US20150105375A1-20150416-C00003.PNG)
![Substituted 2-aza-bicyclo[2.2.2]octane-3-carboxylic acid (benzyl-cyano-methyl)-amides inhibitors of cathepsin c Substituted 2-aza-bicyclo[2.2.2]octane-3-carboxylic acid (benzyl-cyano-methyl)-amides inhibitors of cathepsin c](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/dd49ea19-e685-44b1-bd2c-8be69617a1aa/US20140275159A1-20140918-C00001.png)
![Substituted 2-aza-bicyclo[2.2.2]octane-3-carboxylic acid (benzyl-cyano-methyl)-amides inhibitors of cathepsin c Substituted 2-aza-bicyclo[2.2.2]octane-3-carboxylic acid (benzyl-cyano-methyl)-amides inhibitors of cathepsin c](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/dd49ea19-e685-44b1-bd2c-8be69617a1aa/US20140275159A1-20140918-C00002.png)
![Substituted 2-aza-bicyclo[2.2.2]octane-3-carboxylic acid (benzyl-cyano-methyl)-amides inhibitors of cathepsin c Substituted 2-aza-bicyclo[2.2.2]octane-3-carboxylic acid (benzyl-cyano-methyl)-amides inhibitors of cathepsin c](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/dd49ea19-e685-44b1-bd2c-8be69617a1aa/US20140275159A1-20140918-C00003.png)
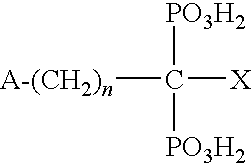
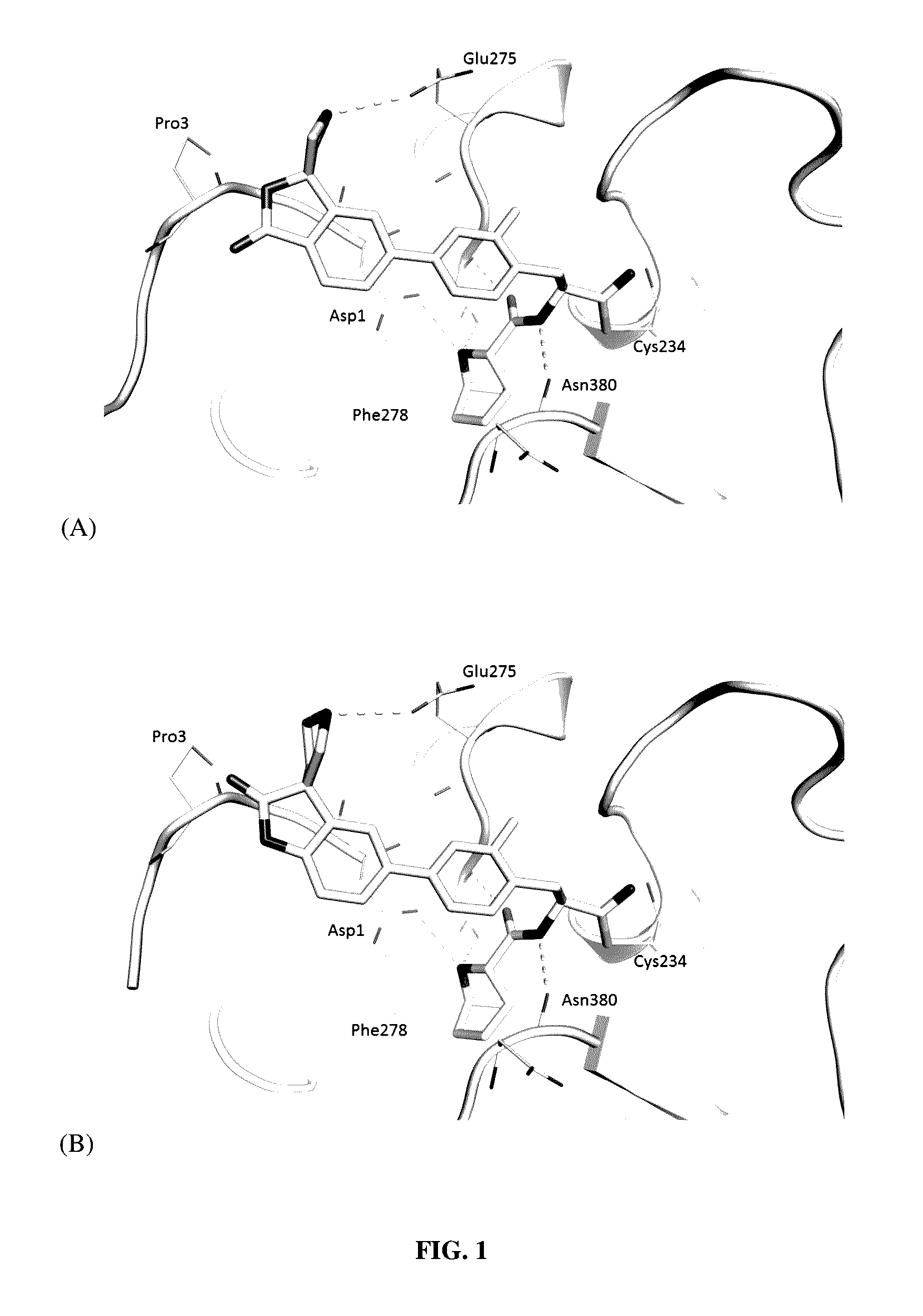




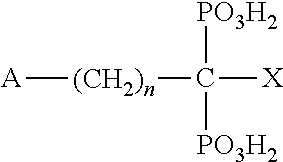
![Methods for treating pulmonary emphysema using substituted 2-aza-bicyclo[2.2.1]heptane-3-carboxylic acid (benzyl-cyano-methyl)-amides inhibitors of cathepsin c Methods for treating pulmonary emphysema using substituted 2-aza-bicyclo[2.2.1]heptane-3-carboxylic acid (benzyl-cyano-methyl)-amides inhibitors of cathepsin c](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2e2e3b25-711e-46b8-83b7-136a29d1dd2e/US20160081982A1-20160324-C00001.PNG)
![Methods for treating pulmonary emphysema using substituted 2-aza-bicyclo[2.2.1]heptane-3-carboxylic acid (benzyl-cyano-methyl)-amides inhibitors of cathepsin c Methods for treating pulmonary emphysema using substituted 2-aza-bicyclo[2.2.1]heptane-3-carboxylic acid (benzyl-cyano-methyl)-amides inhibitors of cathepsin c](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2e2e3b25-711e-46b8-83b7-136a29d1dd2e/US20160081982A1-20160324-C00002.PNG)
![Methods for treating pulmonary emphysema using substituted 2-aza-bicyclo[2.2.1]heptane-3-carboxylic acid (benzyl-cyano-methyl)-amides inhibitors of cathepsin c Methods for treating pulmonary emphysema using substituted 2-aza-bicyclo[2.2.1]heptane-3-carboxylic acid (benzyl-cyano-methyl)-amides inhibitors of cathepsin c](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2e2e3b25-711e-46b8-83b7-136a29d1dd2e/US20160081982A1-20160324-C00003.PNG)