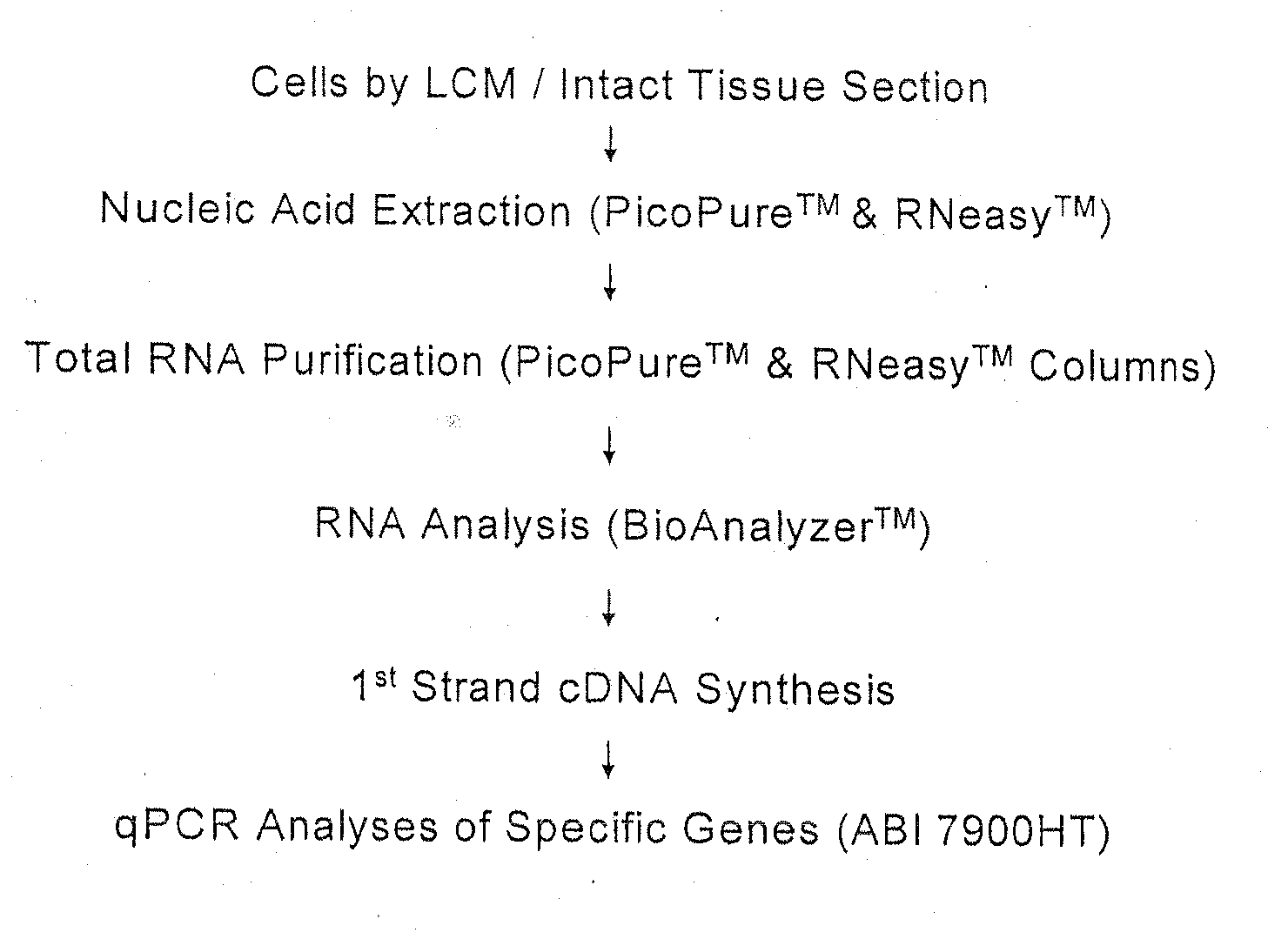
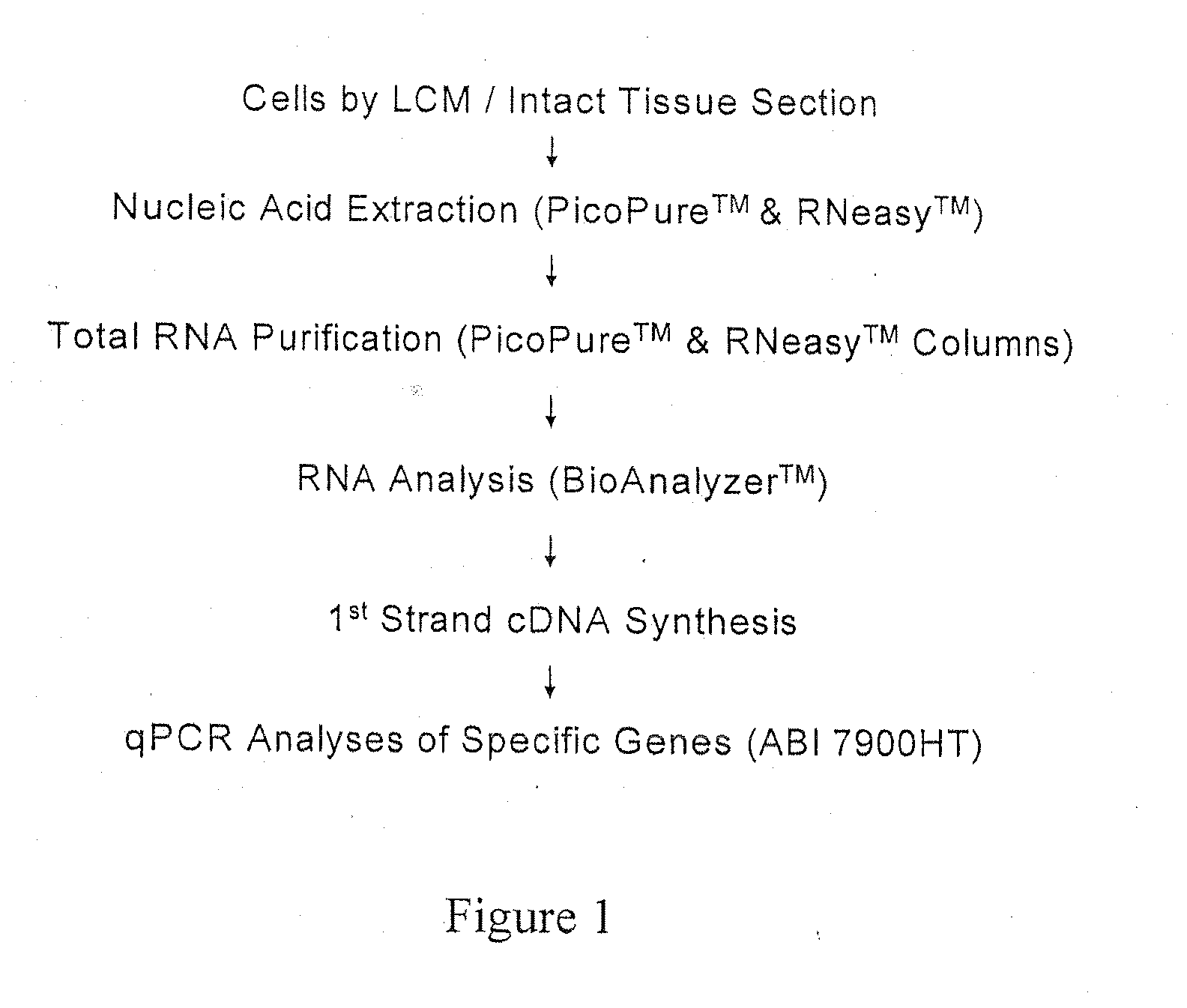
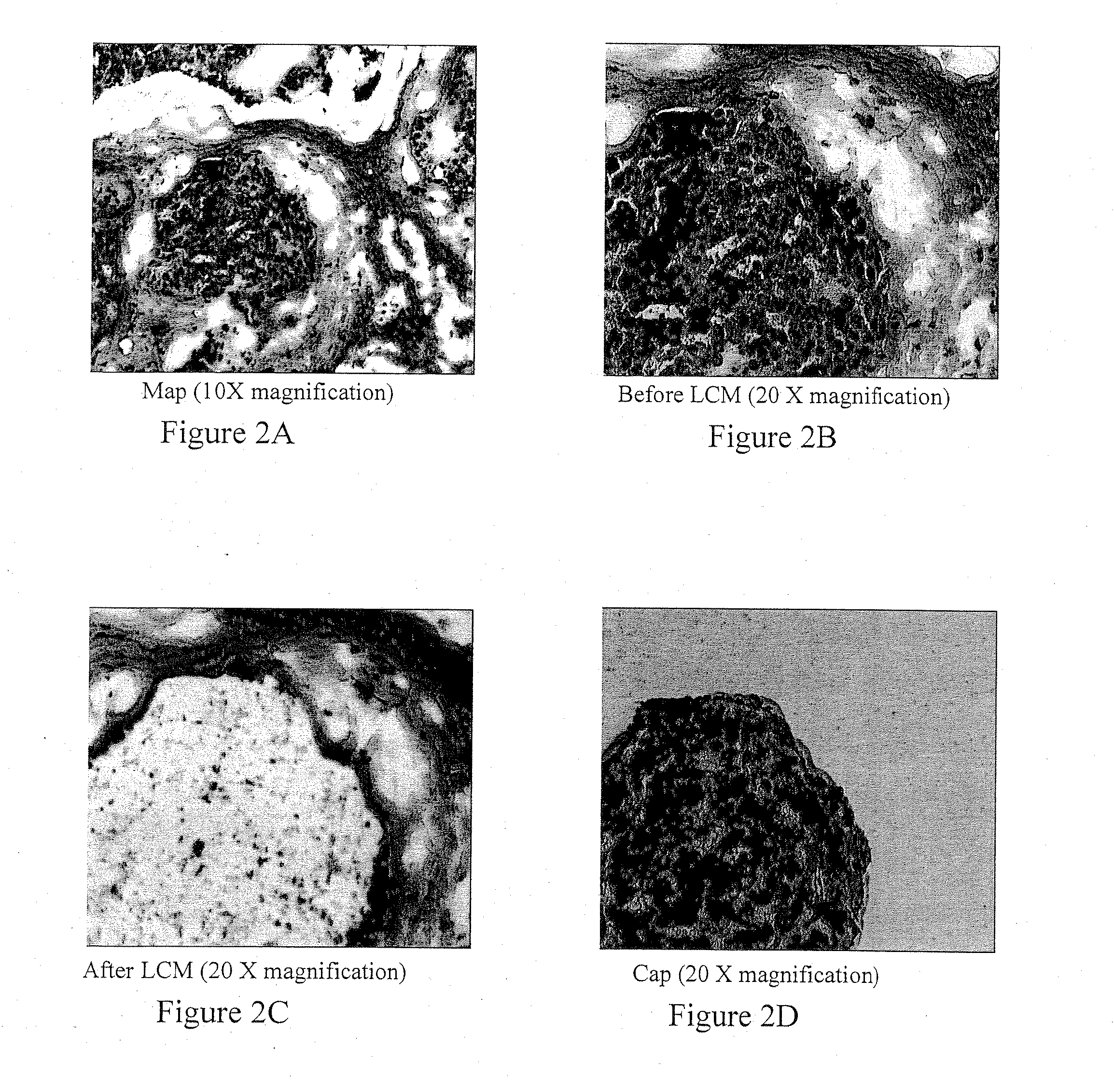
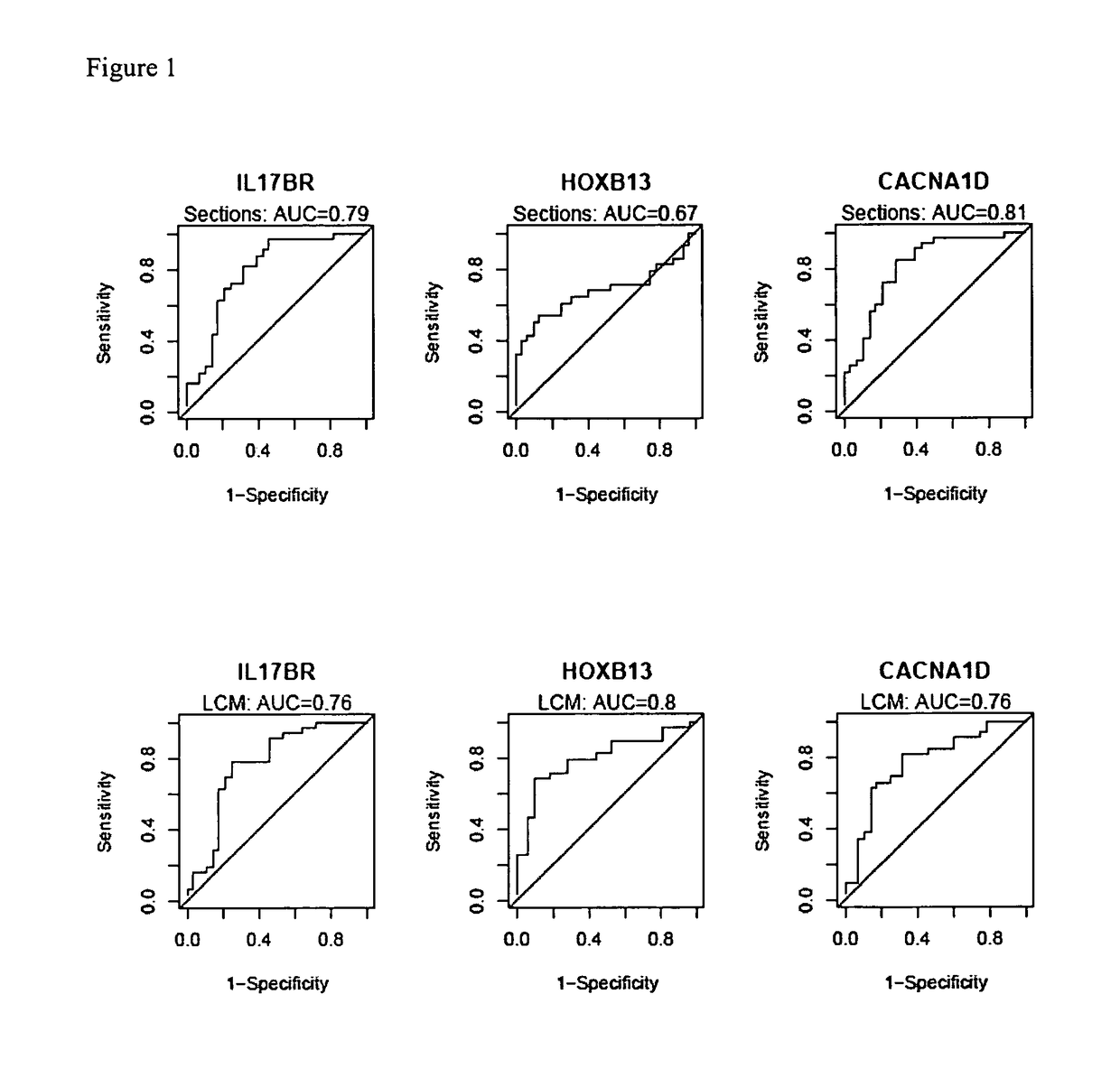
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
32 results about "Breast tissue sample" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
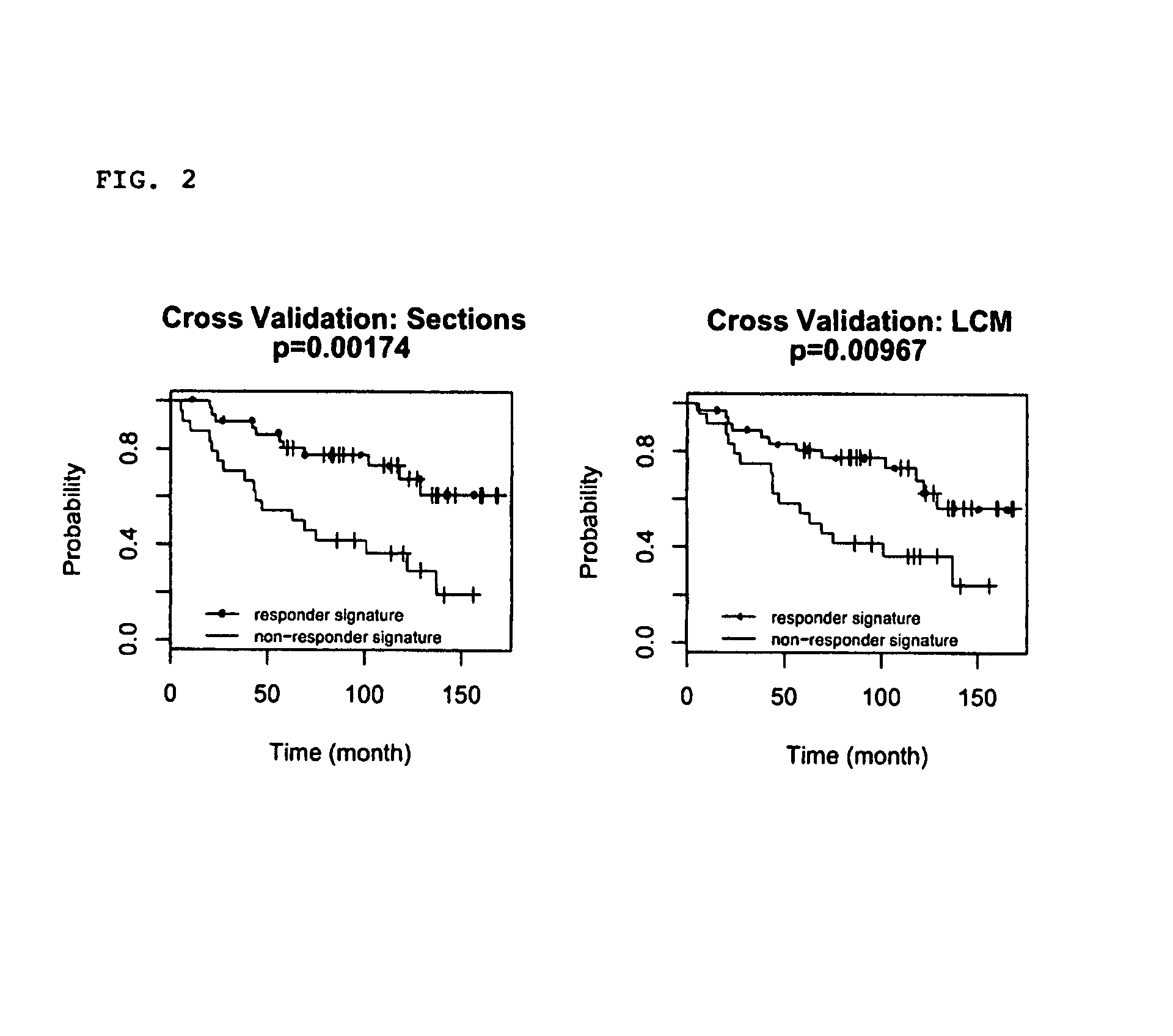
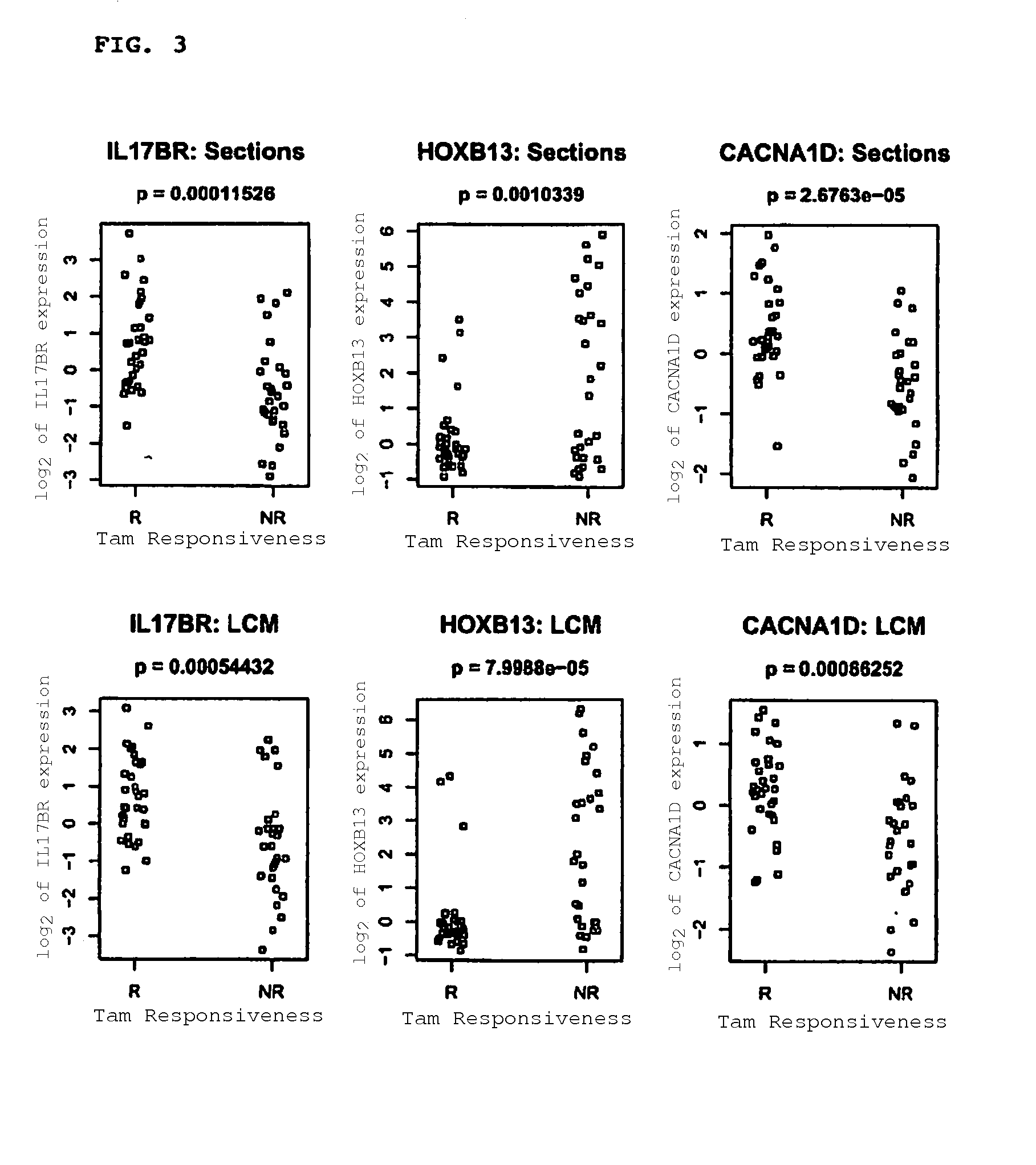
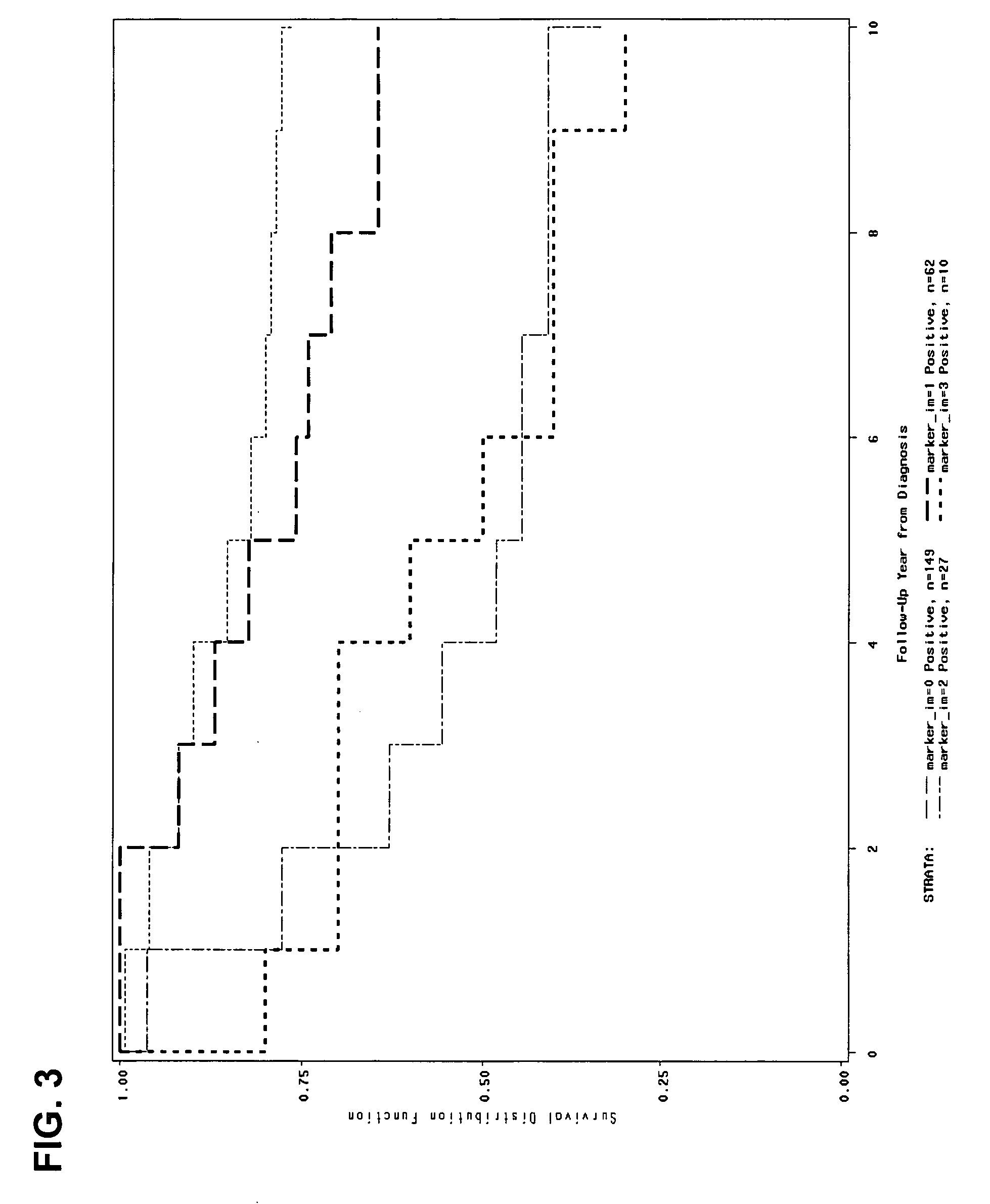
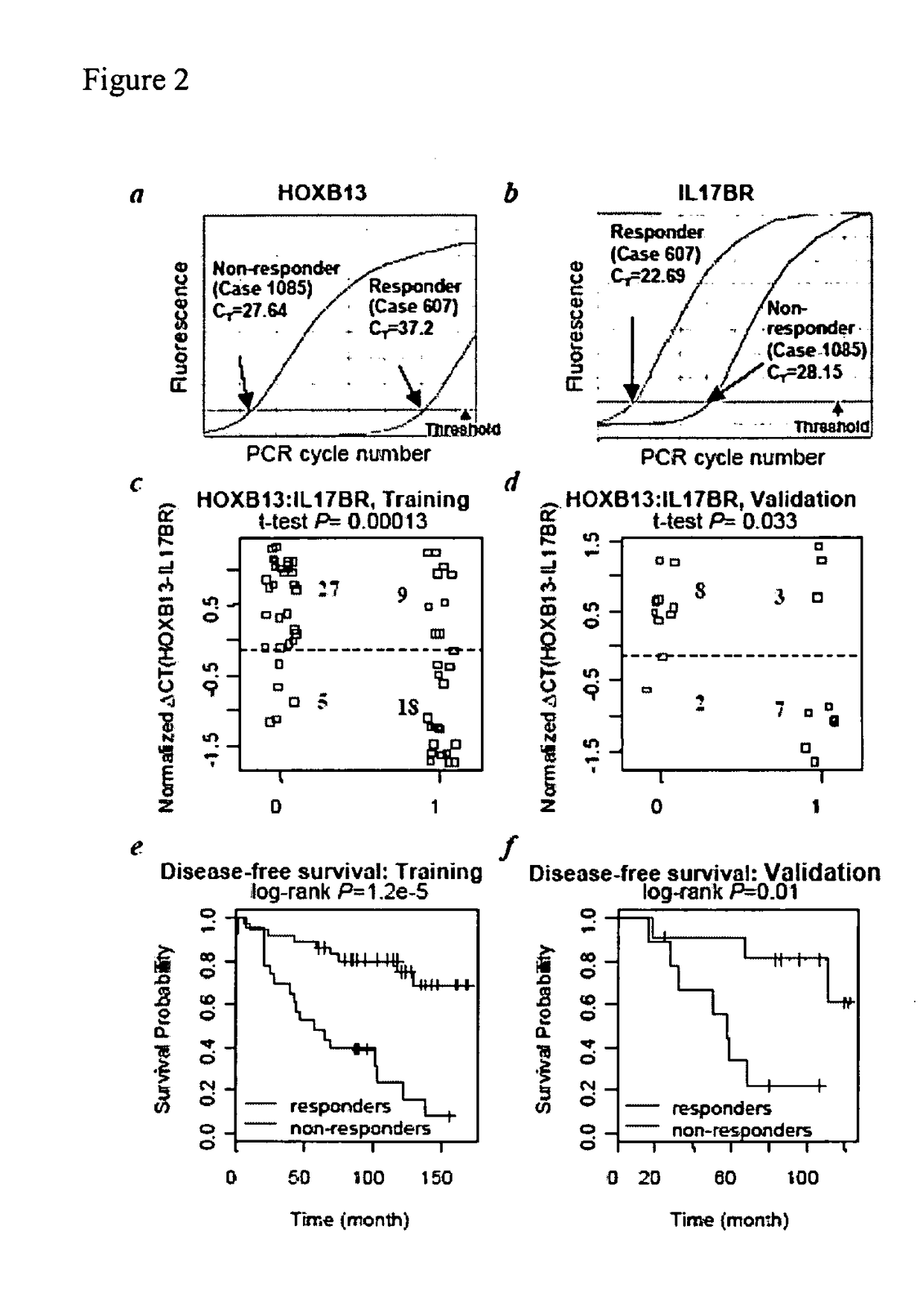
Predicting outcome with tamoxifen in breast cancer
ActiveUS7504214B2Improve predictive performanceBioreactor/fermenter combinationsBiological substance pretreatmentsTamoxifen treatmentOncology
Owner:BIOTHERANOSTICS +1
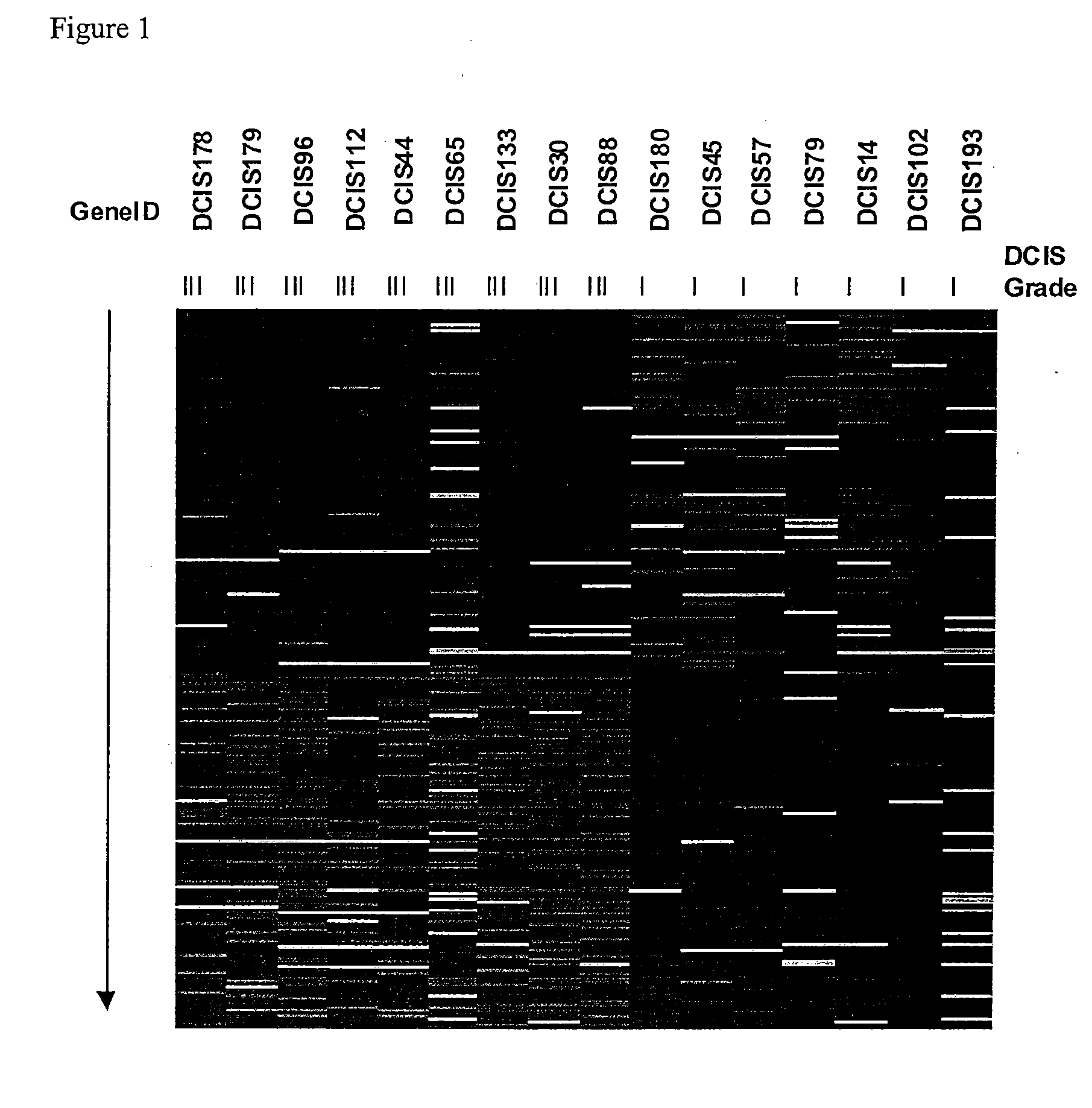
Grading of Breast Cancer
InactiveUS20090092973A1Improve abilitiesAccurate assessmentOrganic active ingredientsSugar derivativesEarly breast cancerOncology
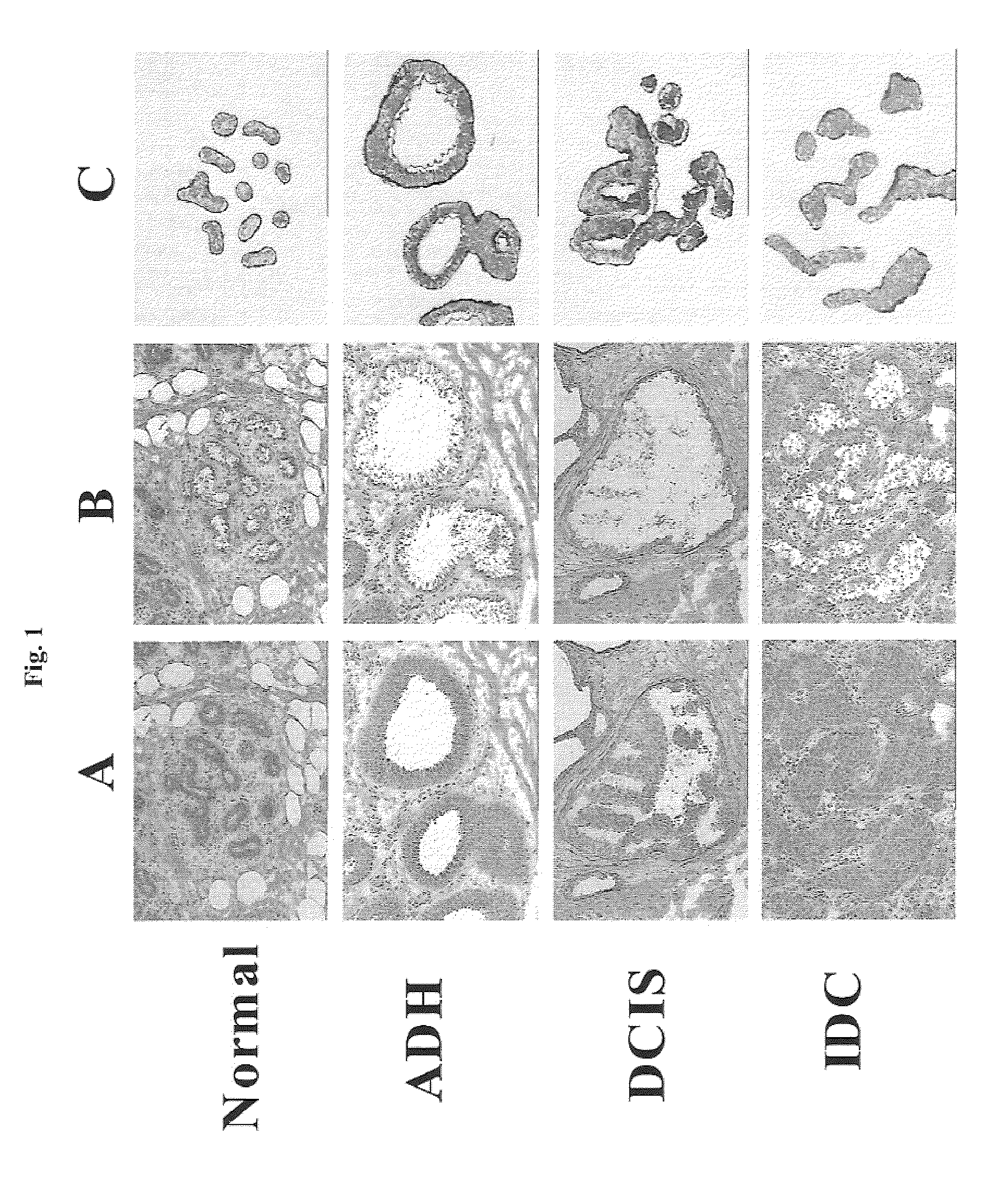
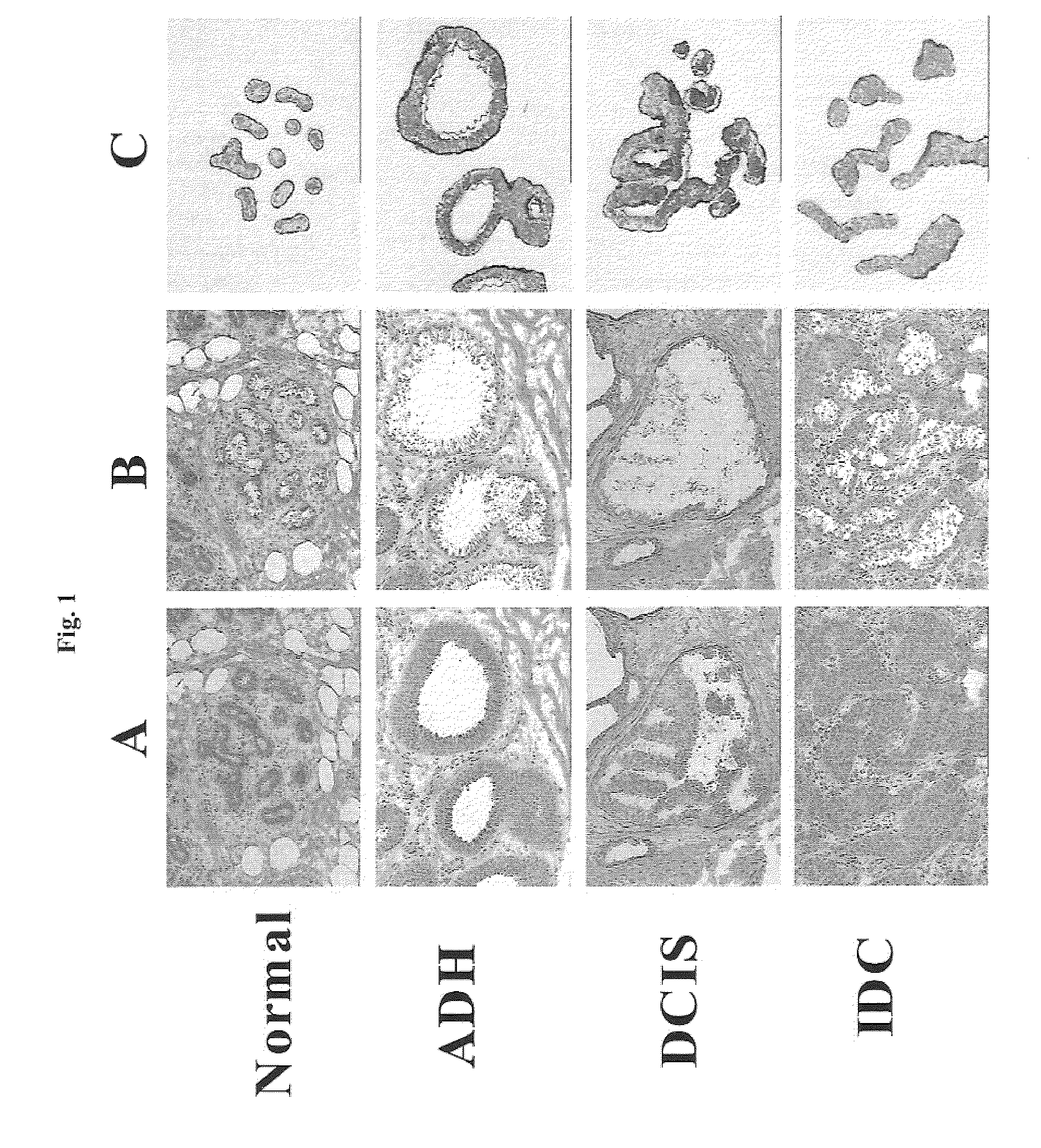
Methods and compositions for the identification of breast cancer grade signatures are provided. The signature profiles are identified based upon multiple sampling of reference breast tissue samples from independent cases of breast cancer and provide a reliable set of molecular criteria for identification of cells as being in one or more particular stages and / or grades of breast cancer.
Owner:THE GENERAL HOSPITAL CORP
Methods and compositions for evaluating breast cancer prognosis
InactiveUS20060063190A1Accurate assessmentImproved prognosisMicrobiological testing/measurementProteomicsLymphatic SpreadNucleic acid hybridisation
Methods and compositions for evaluating the prognosis of a breast cancer patient, particularly an early-stage breast cancer patient, are provided. The methods of the invention comprise detecting expression of at least one, more particularly at least two, biomarker(s) in a body sample, wherein overexpression of the biomarker or a combination of biomarkers is indicative of breast cancer prognosis. In some embodiments, the body sample is a breast tissue sample, particularly a primary breast tumor sample. The biomarkers of the invention are proteins and / or genes whose overexpression is indicative of either a good or bad cancer prognosis. Biomarkers of interest include proteins and genes involved in cell cycle regulation, DNA replication, transcription, signal transduction, cell proliferation, invasion, proteolysis, or metastasis. In some aspects of the invention, overexpression of a biomarker of interest is detected at the protein level using biomarker-specific antibodies or at the nucleic acid level using nucleic acid hybridization techniques.
Owner:TRIPATH IMAGING INC
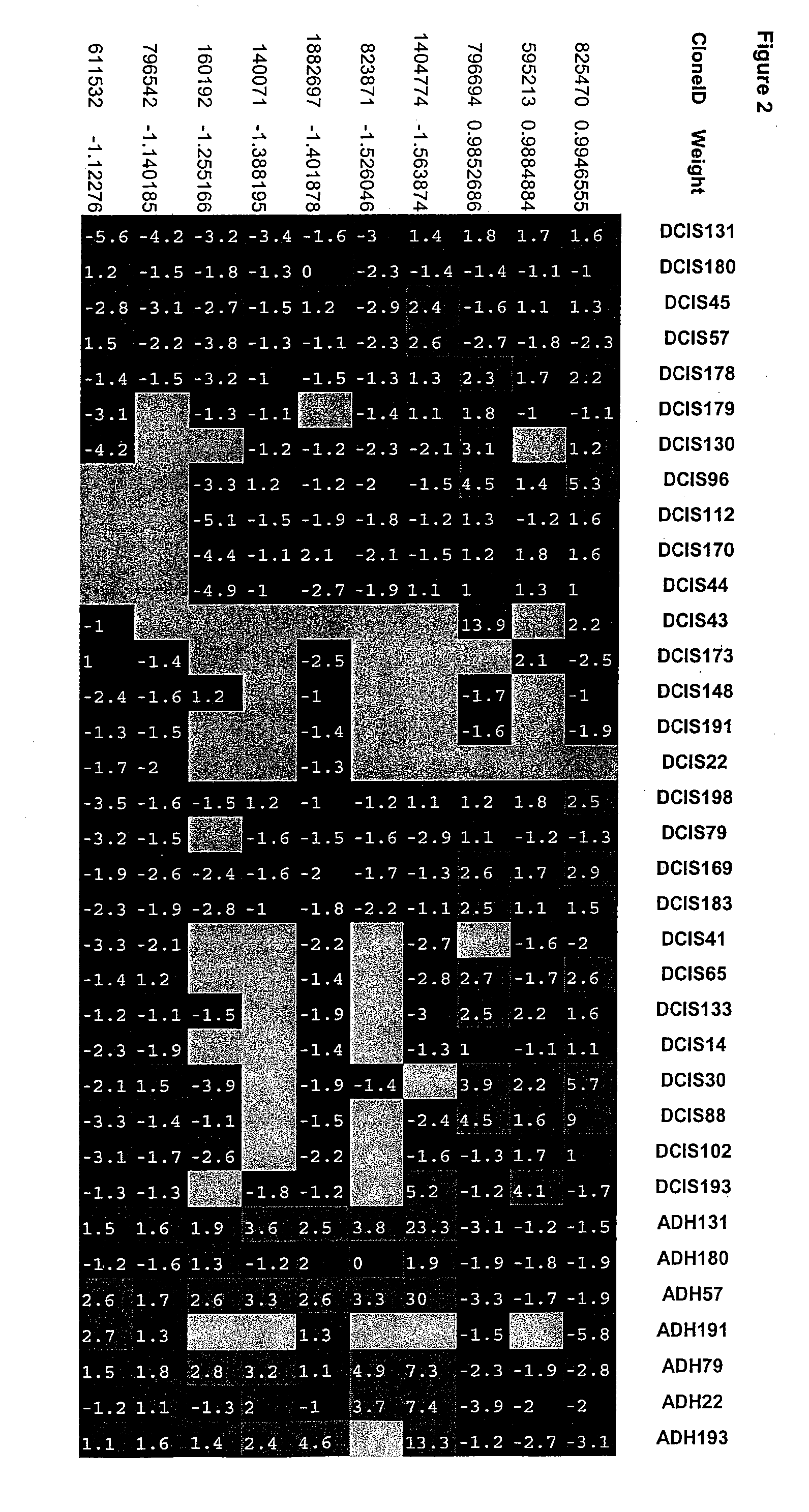
Breast cancer progression signatures
InactiveUS20060234287A1Not affectConfidenceMicrobiological testing/measurementICT adaptationOncologyBreast tissue sample
Methods and compositions for the identification of breast cancer progression signatures are provided. The signature profiles are identified based upon multiple sampling of reference breast tissue samples from independent cases of breast cancer and provide a reliable set of molecular criteria for identification of cells as being in one or more particular stages of breast cancer.
Owner:AVIARADX
Predicting breast cancer treatment outcome
ActiveUS20050239083A1Improve survival outcomeGene expressionBioreactor/fermenter combinationsBiological substance pretreatmentsOncologyBreast tissue sample
Methods and compositions are provided for the identification of expression signatures in ER+ breast cancer cases, where the signatures correlate with responsiveness, or lack thereof, to treatment with tamoxifen or another antiestrogen agent against breast cancer The signature profiles are identified based upon sampling of reference breast tissue samples from independent cases of breast cancer and provide a reliable set of molecular criteria for predicting the efficacy of treating a subject with breast cancer with tamoxifen or another antiestrogen agent against breast cancer. Additional methods and compositions are provided for predicting responsiveness to tamoxifen or another antiestrogen agent against breast cancer in cases of breast cancer by use of three biomarkers. Two biomarkers display increased expression correlated with tamoxifen response while the third biomarker displays decreased expression correlated with tamoxifen response.
Owner:THE GENERAL HOSPITAL CORP +1
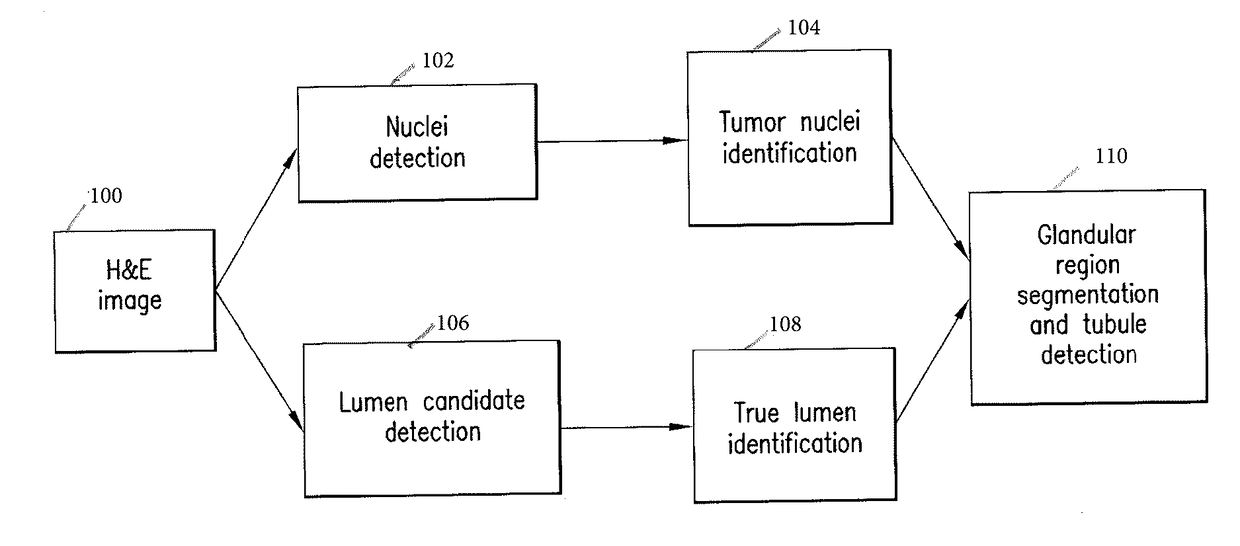
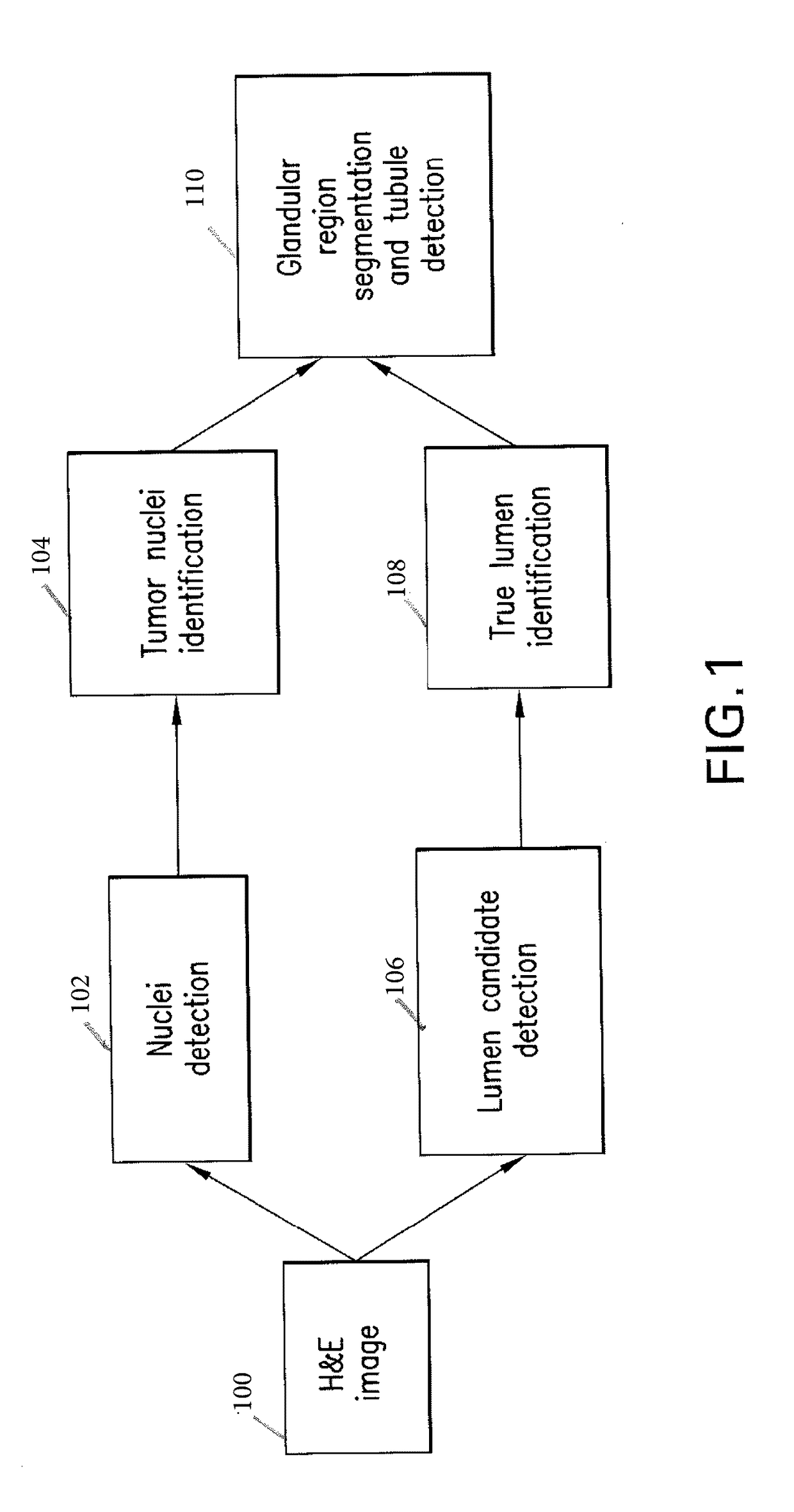
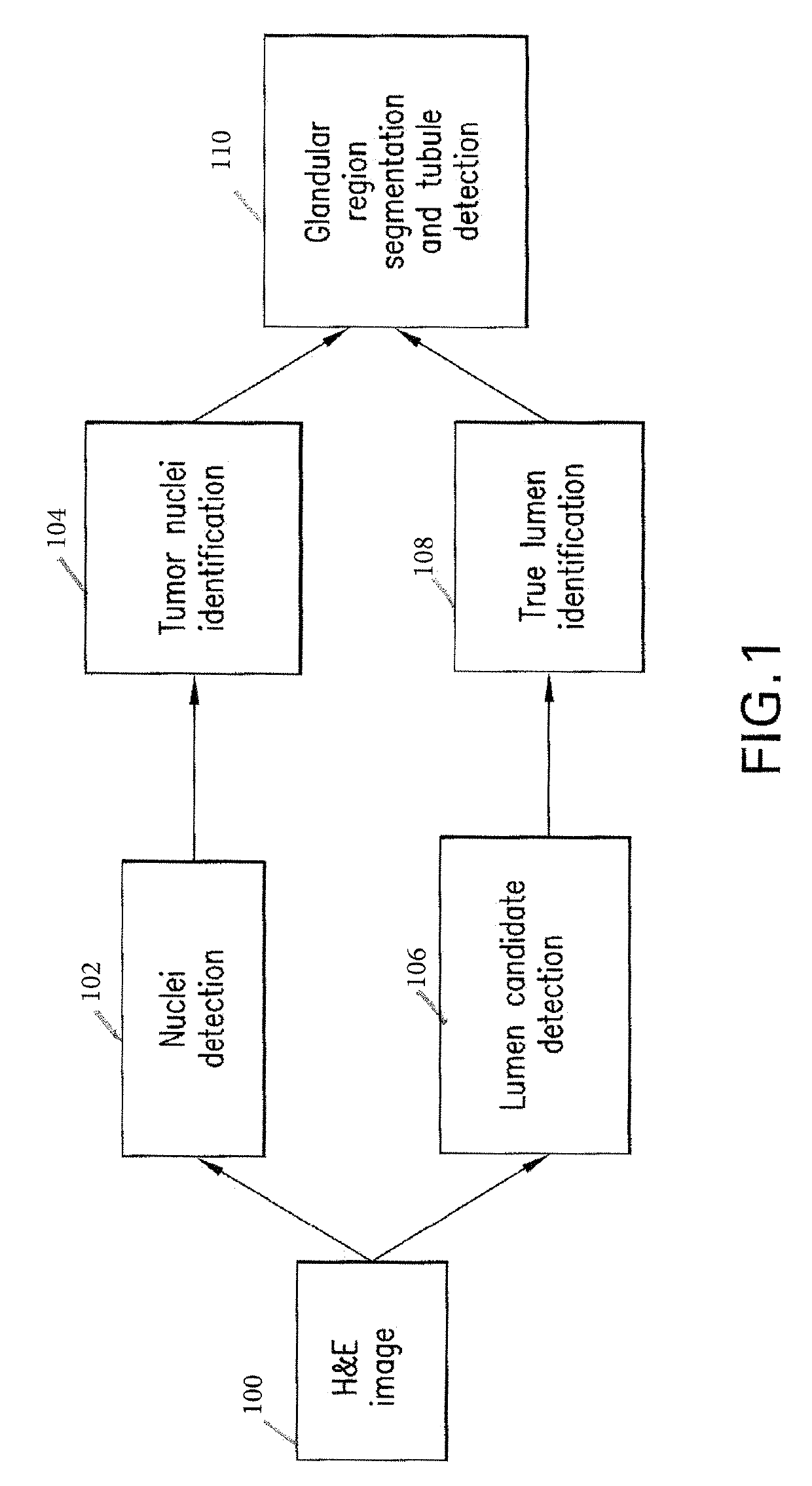
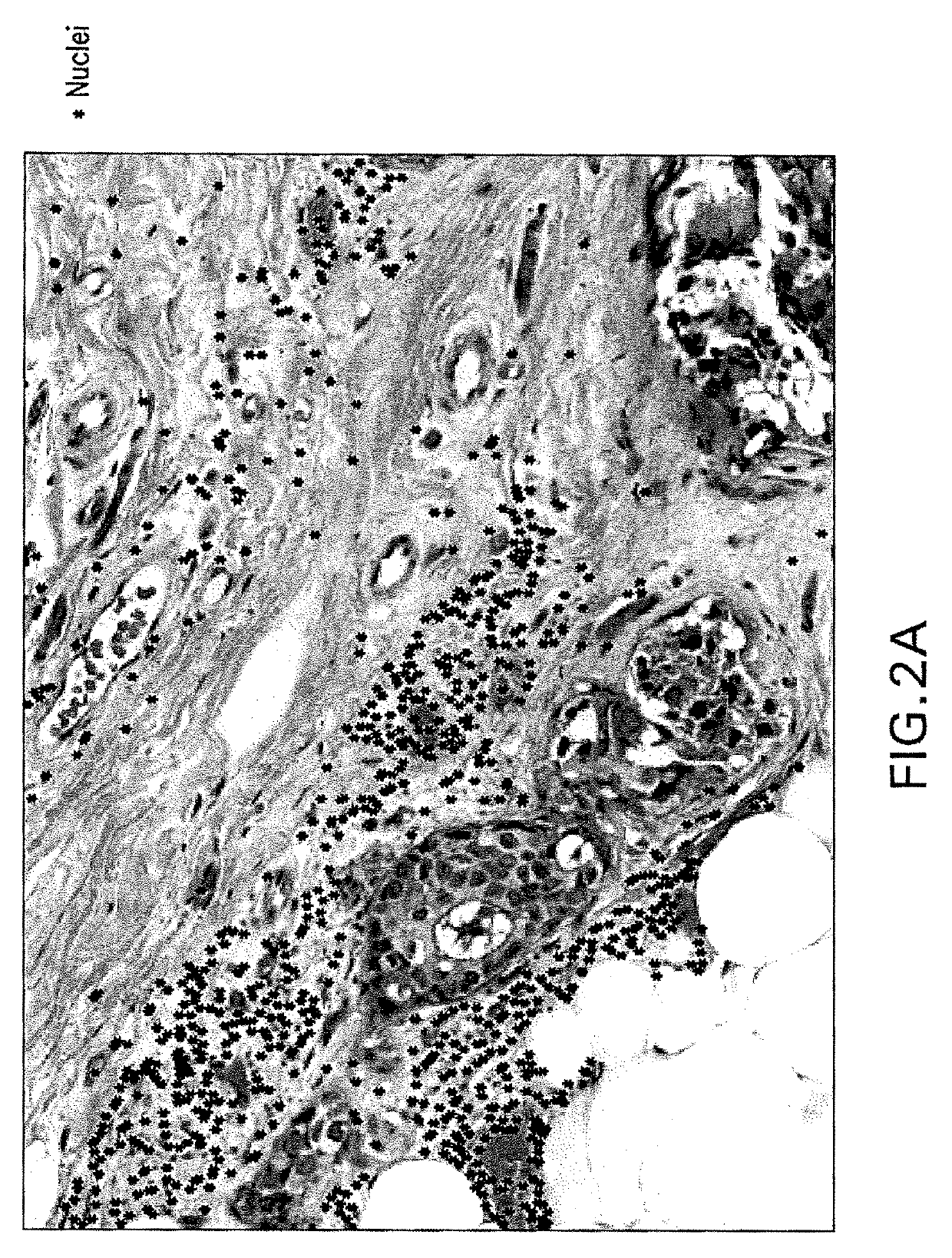
Automatic glandular and tubule detection in histological grading of breast cancer
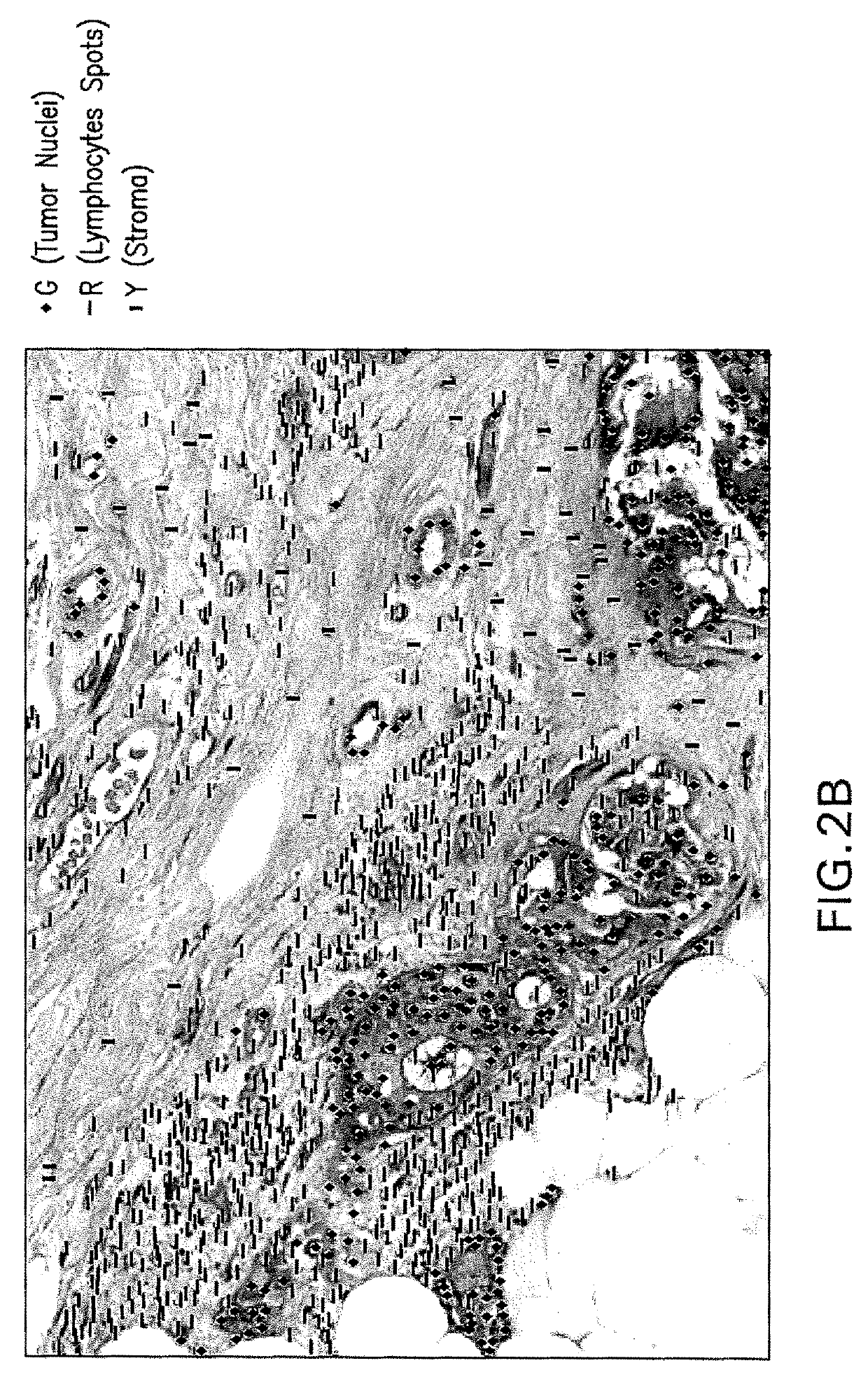
Methods, systems, and apparatuses for automatically identifying glandular regions and tubule regions in a breast tissue sample are provided. An image of breast tissue is analyzed to detect nuclei and lumen candidates, identify tumor nuclei and true lumen from the candidates, and group tumor nuclei with neighboring tumor nuclei and lumina to define tubule glandular regions and non-tubule glandular regions of the image. Learnt supervised classifiers, such as random forest classifiers, can be applied to identify and classify the tumor nuclei and true lumina. Graph-cut methods can be applied to group the tumor nuclei and lumina and to define the tubule glandular regions and non-tubule glandular regions. The analysis can be applied to whole slide images and can resolve tubule areas with multiple layers of nuclei.
Owner:VENTANA MEDICAL SYST INC
Diagnosis and treatment of breast cancer
InactiveUS20060154267A1Improve survival outcomeGene expressionMechanical/radiation/invasive therapiesData processing applicationsCurative effectOncology
Methods and compositions are provided for the identification of expression signatures in ER+ breast cancer cases, where the signatures correlate with responsiveness, or lack thereof, to treatment with tamoxifen or another antiestrogen agent against breast cancer The signature profiles are identified based upon sampling of reference breast tissue samples from independent cases of breast cancer and provide a reliable set of molecular criteria for predicting the efficacy of treating a subject with breast cancer with tamoxifen or another antiestrogen agent against breast cancer. Additional methods and compositions are provided for predicting responsiveness to tamoxifen or another antiestrogen agent against breast cancer in cases of breast cancer by use of multiple biomarkers. Two biomarkers display increased expression correlated with tamoxifen response while two other biomarkers display decreased expression correlated with tamoxifen response.
Owner:AVIARADX +1
Methods for a predictive diagnostic test for tamoxifen
InactiveUS20090155767A1Microbiological testing/measurementDisease diagnosisBreast tissue sampleDiagnostic Trial
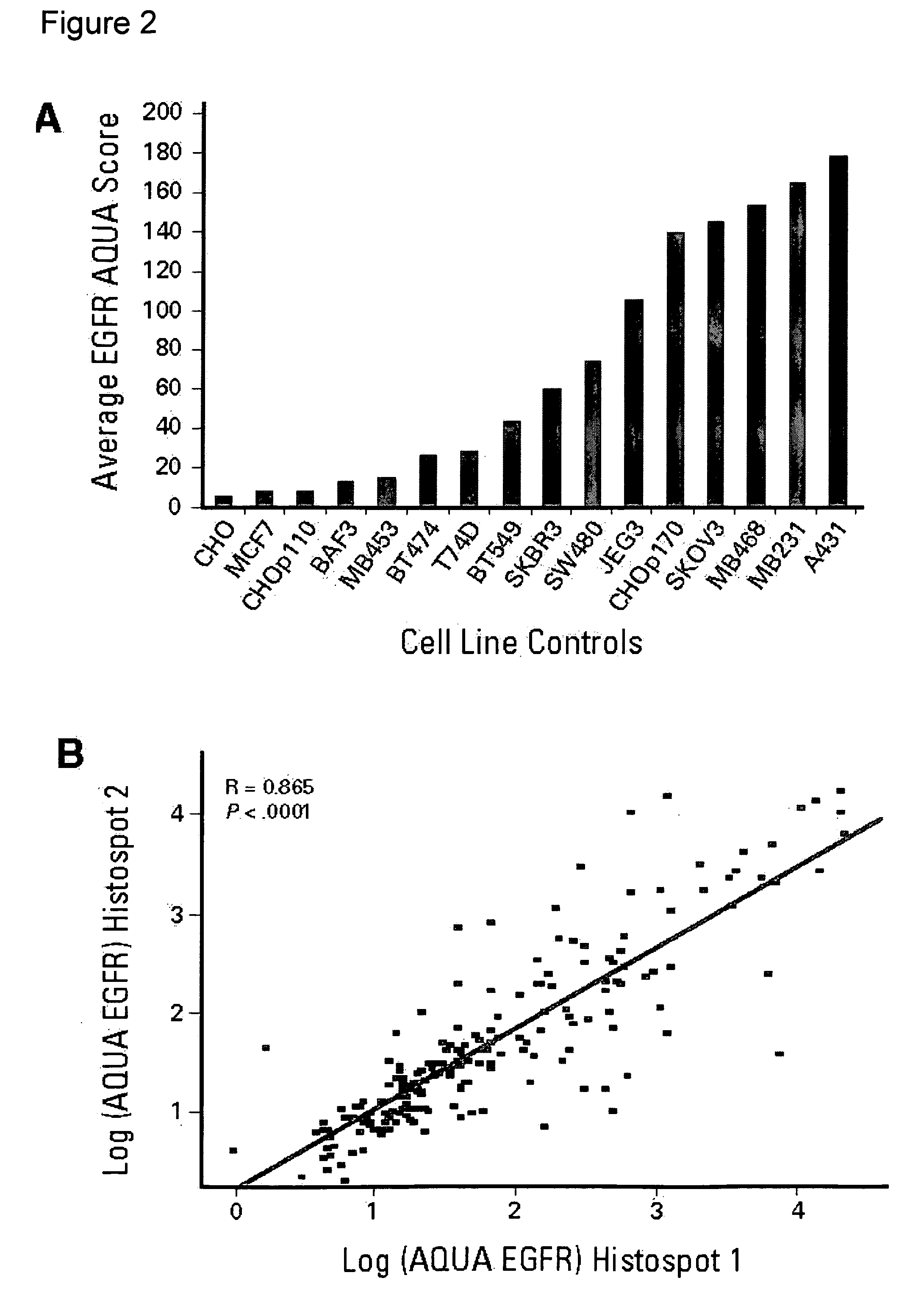
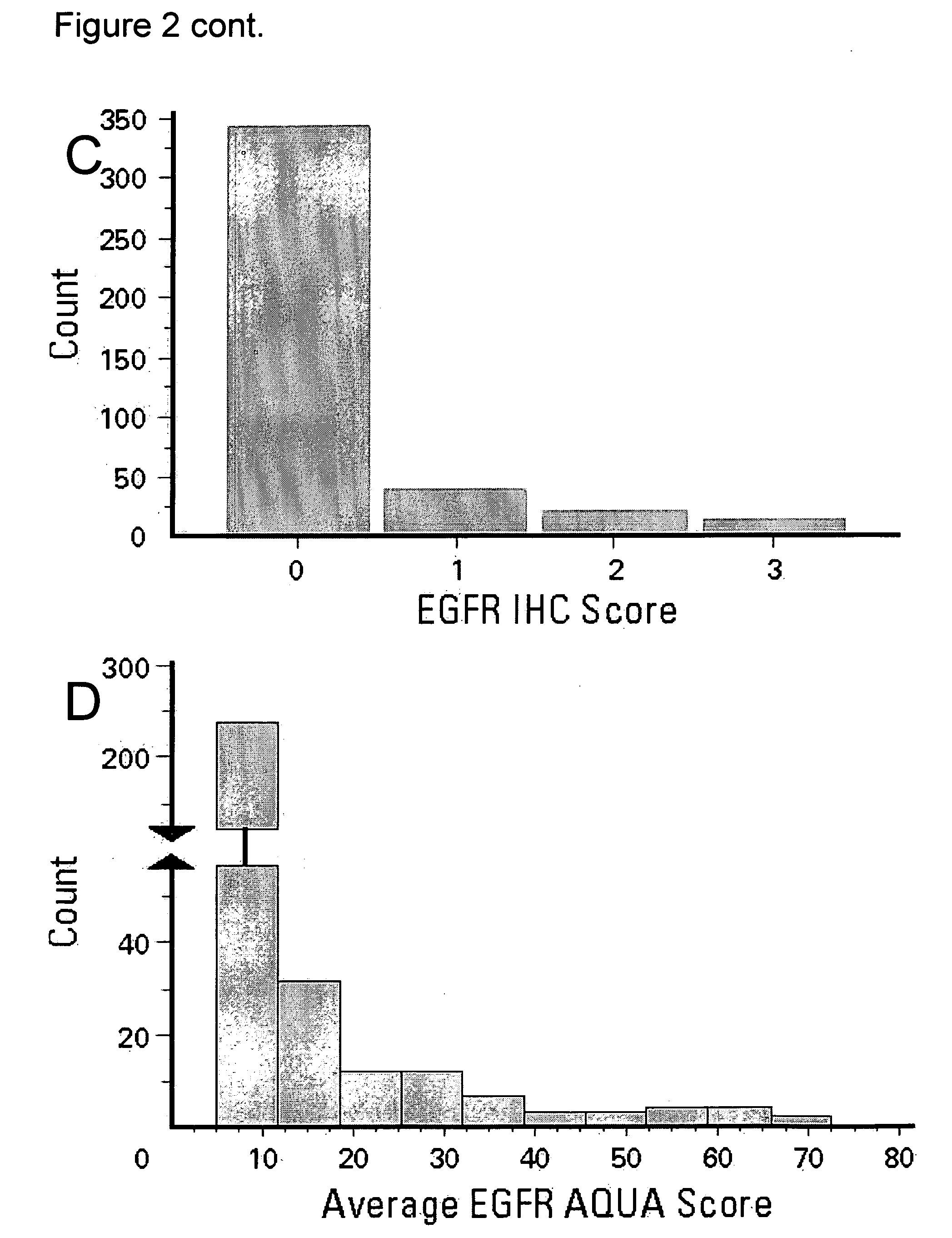
A method for determining the likelihood that a therapy involving administration of tamoxifen to a patient afflicted with an estrogen receptor positive breast cancer will provide a therapeutic benefit to the patient which comprises determining the level of expression of epidermal growth factor receptor present within a non-nuclear compartment in cells present in a breast tissue sample from the patient; and comparing the level of expression so obtained to a predetermined level of expression wherein the likelihood the therapy will provide a therapeutic benefit to the patient is greater if the level of expression in step a) is less than the predetermined level of expression.
Owner:RIMM DAVID L +2
Predicting breast cancer treatment outcome
InactiveCN1969047ABioreactor/fermenter combinationsBiological substance pretreatmentsPharmaceutical drugOncology
Methods and compositions are provided for the identification of expression signatures in ER+ breast cancer cases, where the signatures correlate with responsiveness, or lack thereof, to treatment with tamoxifen or another antiestrogen agent against breast cancer. The signature profiles are identified based upon sampling of reference breast tissue samples from independent cases of breast cancer and provide a reliable set of molecular criteria for predicting the efficacy of treating a subject with breast cancer with tamoxifen or another antiestrogen agent against breast cancer. Additional methods and compositions are provided for predicting responsiveness to tamoxifen or another antiestrogen agent against breast cancer in cases of breast cancer by use of multiple biomarkers. Two biomarkers display increased expression correlated with tamoxifen response while two other biomarkers display decreased expression correlated with tamoxifen response.
Owner:阿克丘勒斯生物科学股份有限公司
Grading of breast cancer
InactiveUS7930105B2Accurate assessmentDetermining prognosisOrganic active ingredientsSugar derivativesEarly breast cancerOncology
Methods and compositions for the identification of breast cancer grade signatures are provided. The signature profiles are identified based upon multiple sampling of reference breast tissue samples from independent cases of breast cancer and provide a reliable set of molecular criteria for identification of cells as being in one or more particular stages and / or grades of breast cancer.
Owner:THE GENERAL HOSPITAL CORP
Novel biomarker for the prognosis of breast cancer
InactiveUS20110097732A1Improved prognosisSugar derivativesImmunoglobulins against animals/humansEarly breast cancerOncology
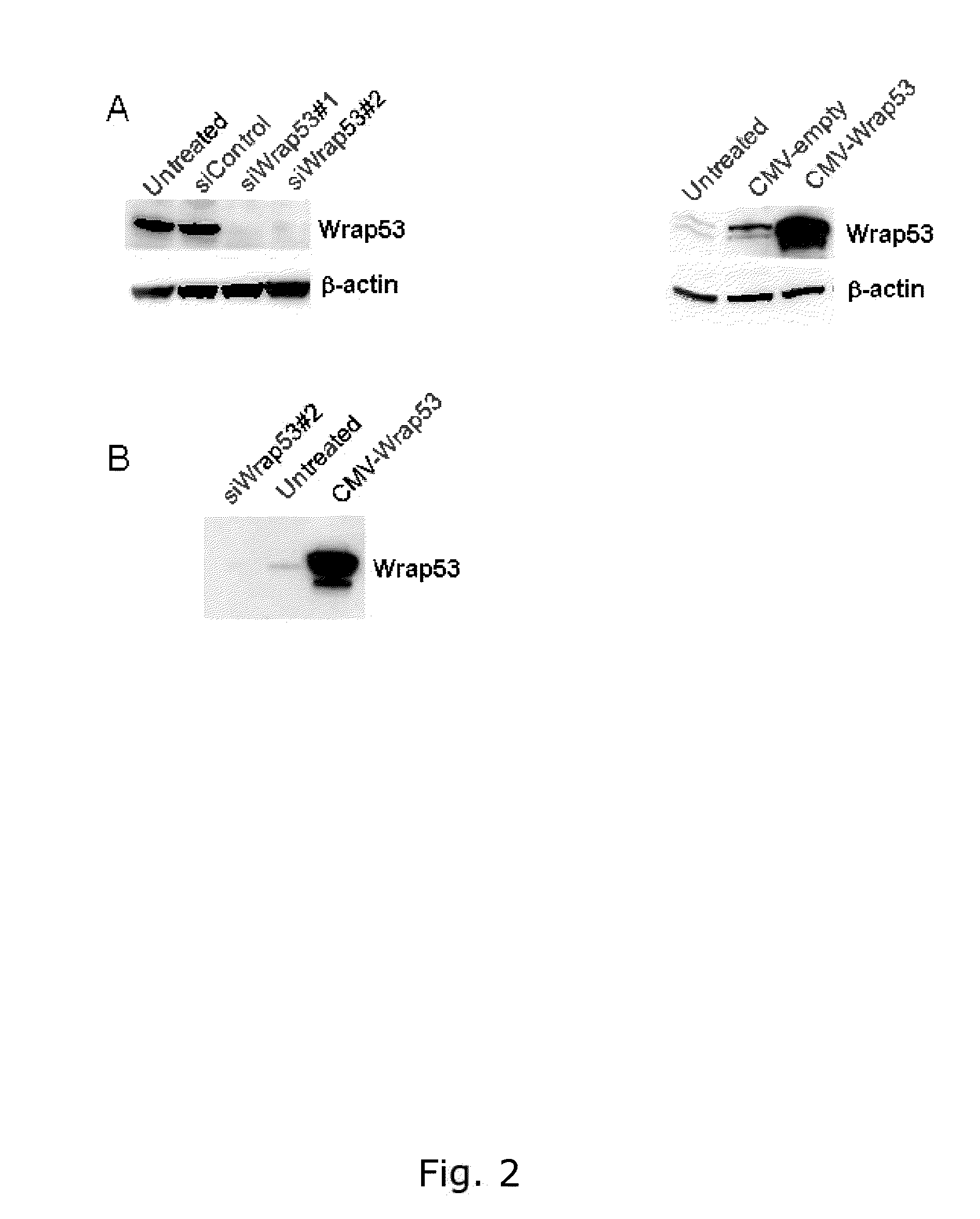
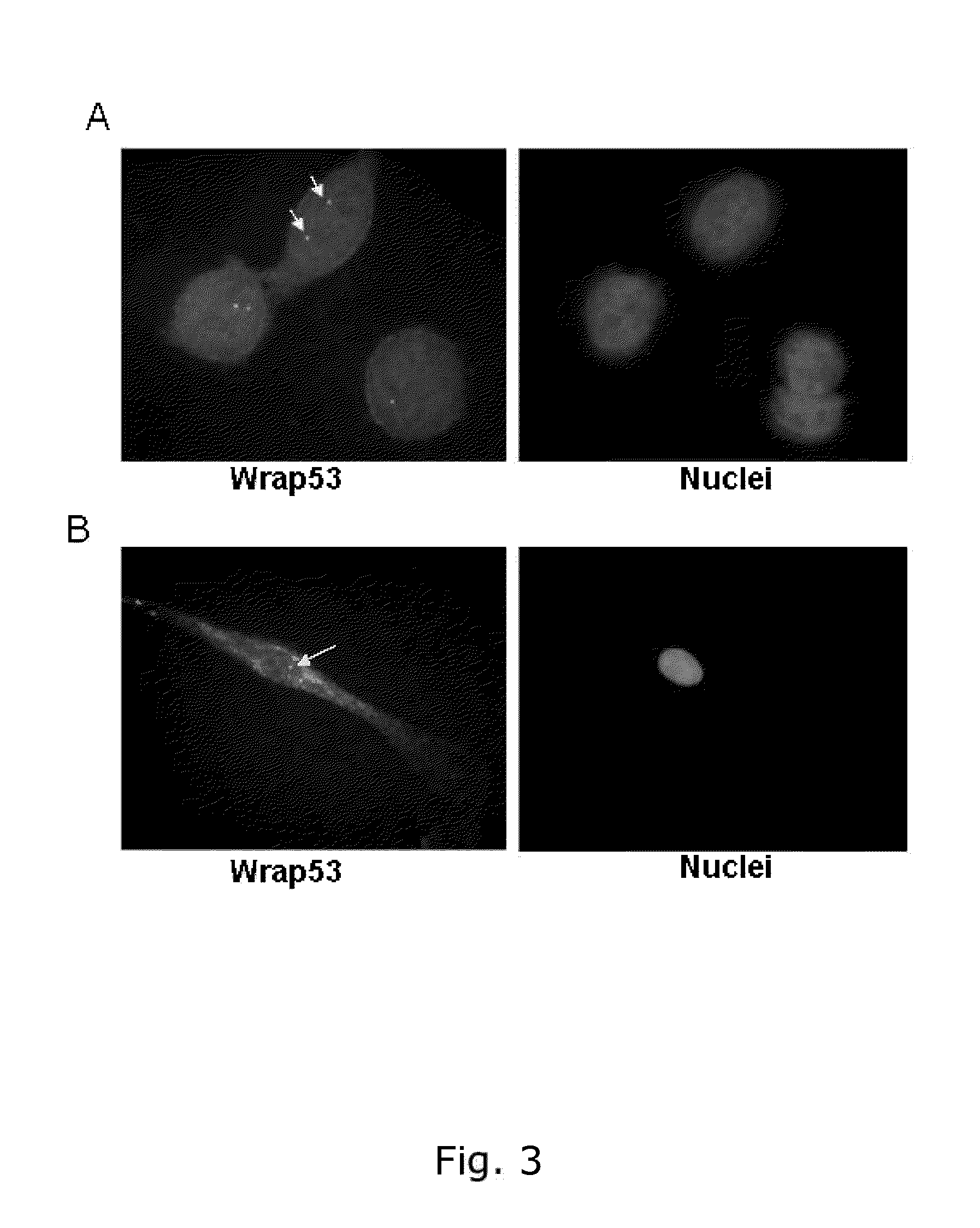
The present invention relates to methods of determining the predilection for survival for an individual having breast cancer comprising: obtaining a breast tissue sample from said individual, measuring the amount of Wrap53 in the nucleus of cells in said breast tissue sample, and / or measuring the amount of Wrap53 in the cytoplasm of cells in said breast tissue sample, and wherein both the nuclear levels, the cytoplasmic levels and / or the ratio between the nuclear and cytoplasmic levels of Wrap53 may be used alone or in combination in breast cancer prognosis. The invention furthermore relates to antibodies and kits relating to Wrap53.
Owner:MEDINNOVA AS
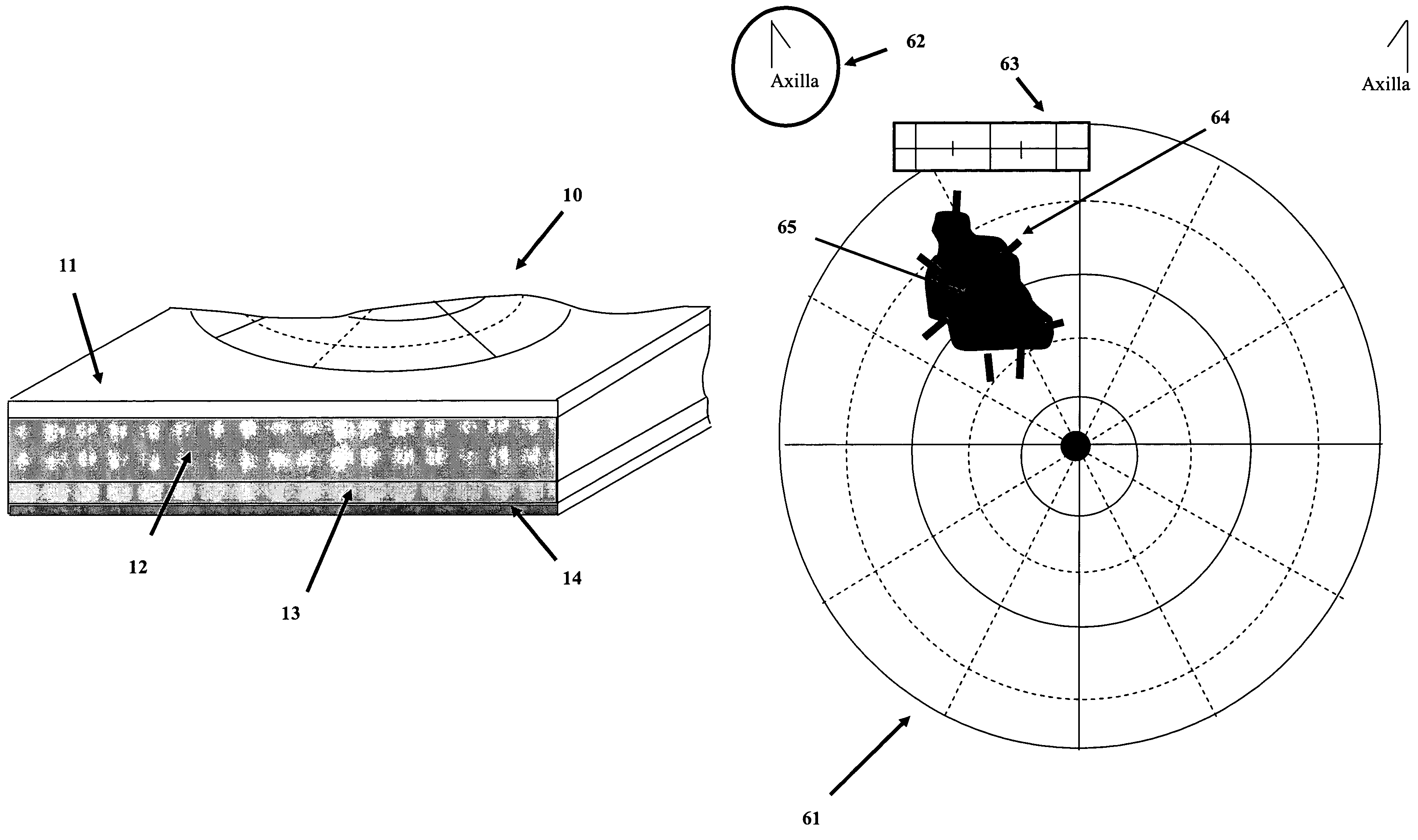
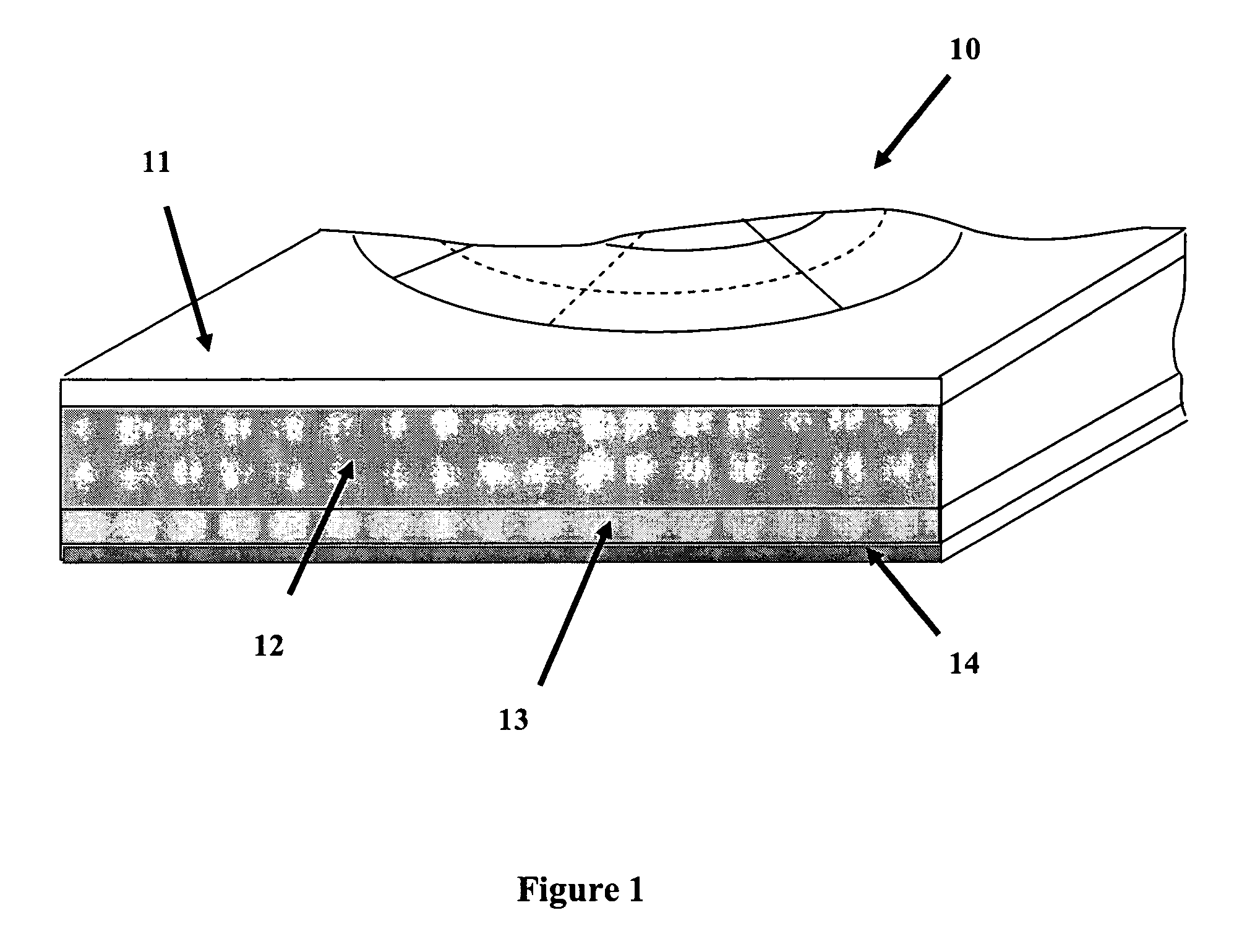
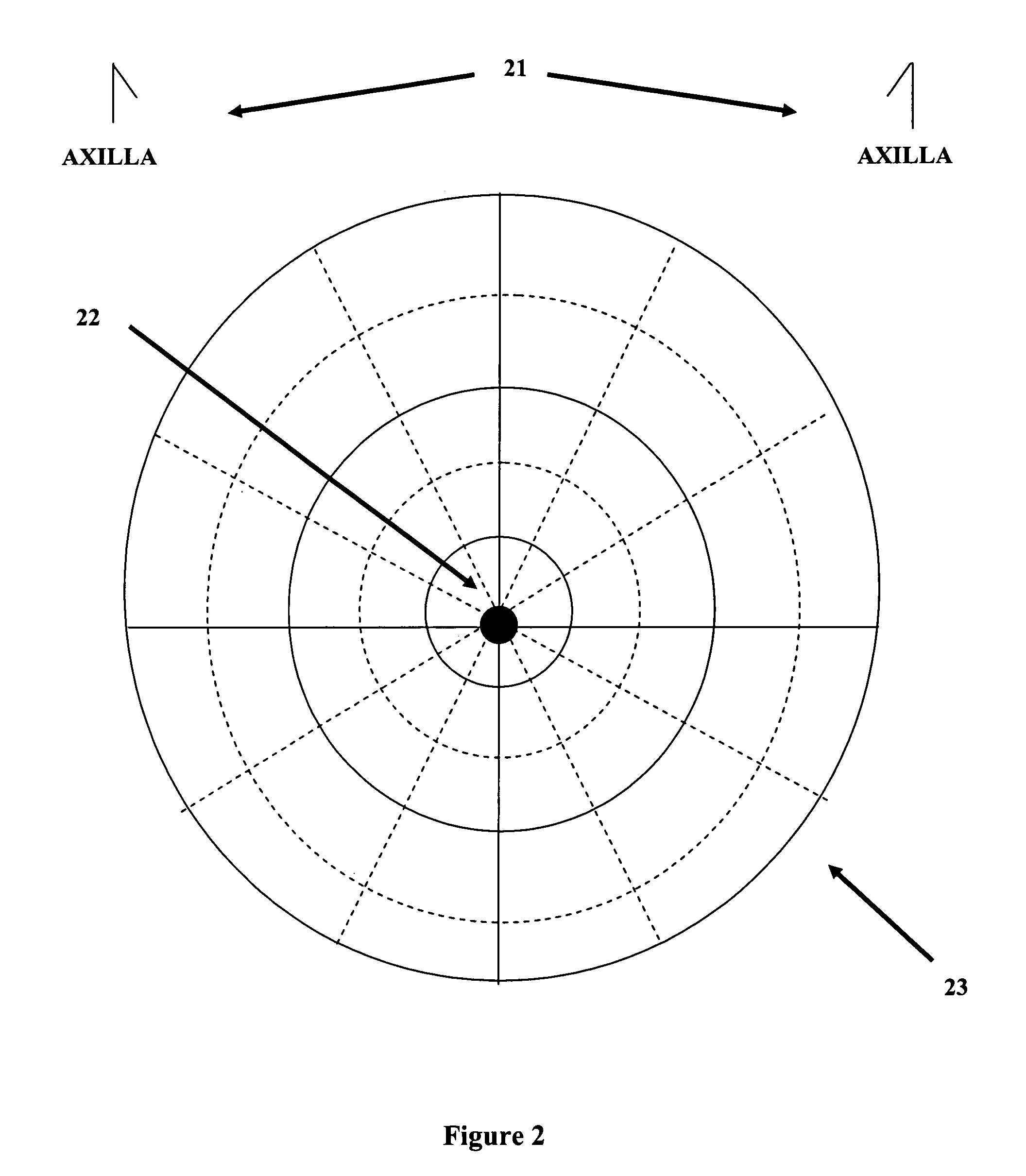
Gross pathology breast map
A system and method to position and orient an excised breast tissue specimen on a support structure in a duplicate anatomic orientation relative to the operated breast is provided. One embodiment of the system includes an anatomically representative map of the breast under investigation. The system allows a surgeon to suture, ink, or otherwise fix the excised specimen on the map in accordance to its original position and orientation in the operated breast or organ. A radiogram of the excised specimen can be taken that shows the location of calcifications on a grid with radiopaque markers. The system allows a pathologist to cut through the specimen utilizing the map as a template in order to perform histological analysis and correlate the position and orientation of each slice to its anatomic position in the breast and thereby direct a precise and anatomically accurate re-excision by the surgeon if so required.
Owner:XOFT INC +1
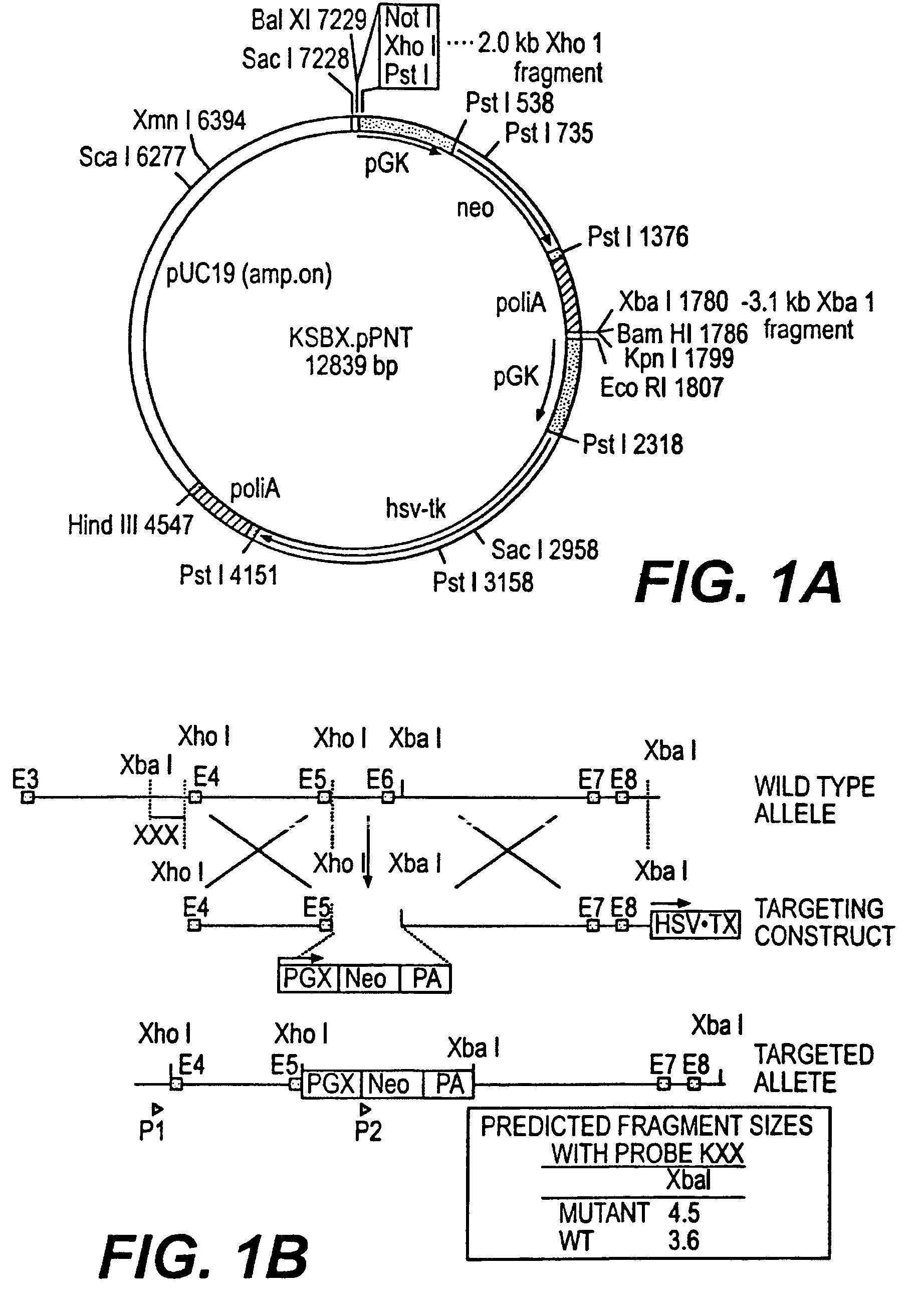
Knockout mouse for the tumor suppressor gene ANX7
InactiveUS7504223B2Inhibit cell proliferationVirusesMicrobiological testing/measurementKnockout animalOncology
This invention provides methods, including a method of assessing the prognosis of a breast cancer patient, comprising assaying for loss of heterozygosity at the 10q21 region of the genome of the patient, a method of identifying a probability that a patient with breast cancer has metastasized breast cancer, a method of determining a survival probability of a patient with breast cancer, and a method of identifying a probability that a patient with prostate cancer has a severe form of prostate cancer. This invention also provides assay complexes, including assay complexes which comprise at least one prostate tissue sample or tissue sample extract, an antibody that specifically binds ANX7, and a label, or which comprise at least one breast tissue sample or tissue sample extract, an antibody that specifically binds ANX7, and a label.
Owner:THE HENRY M JACKSON FOUND FOR THE ADVANCEMENT OF MILITARY MEDICINE INC
Markers for identifying breast cancer treatment modalities
InactiveUS20140011695A1Microbiological testing/measurementLibrary screeningEndocrine therapyOncology
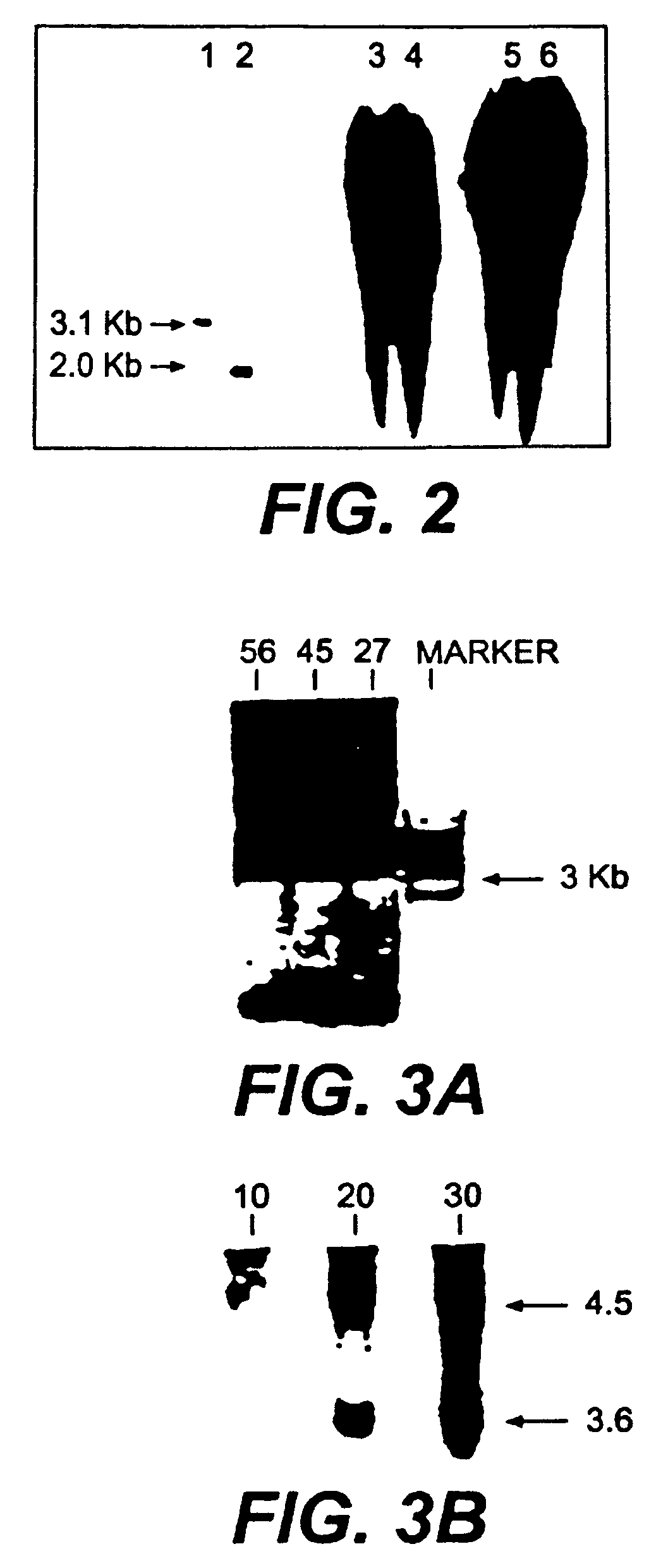
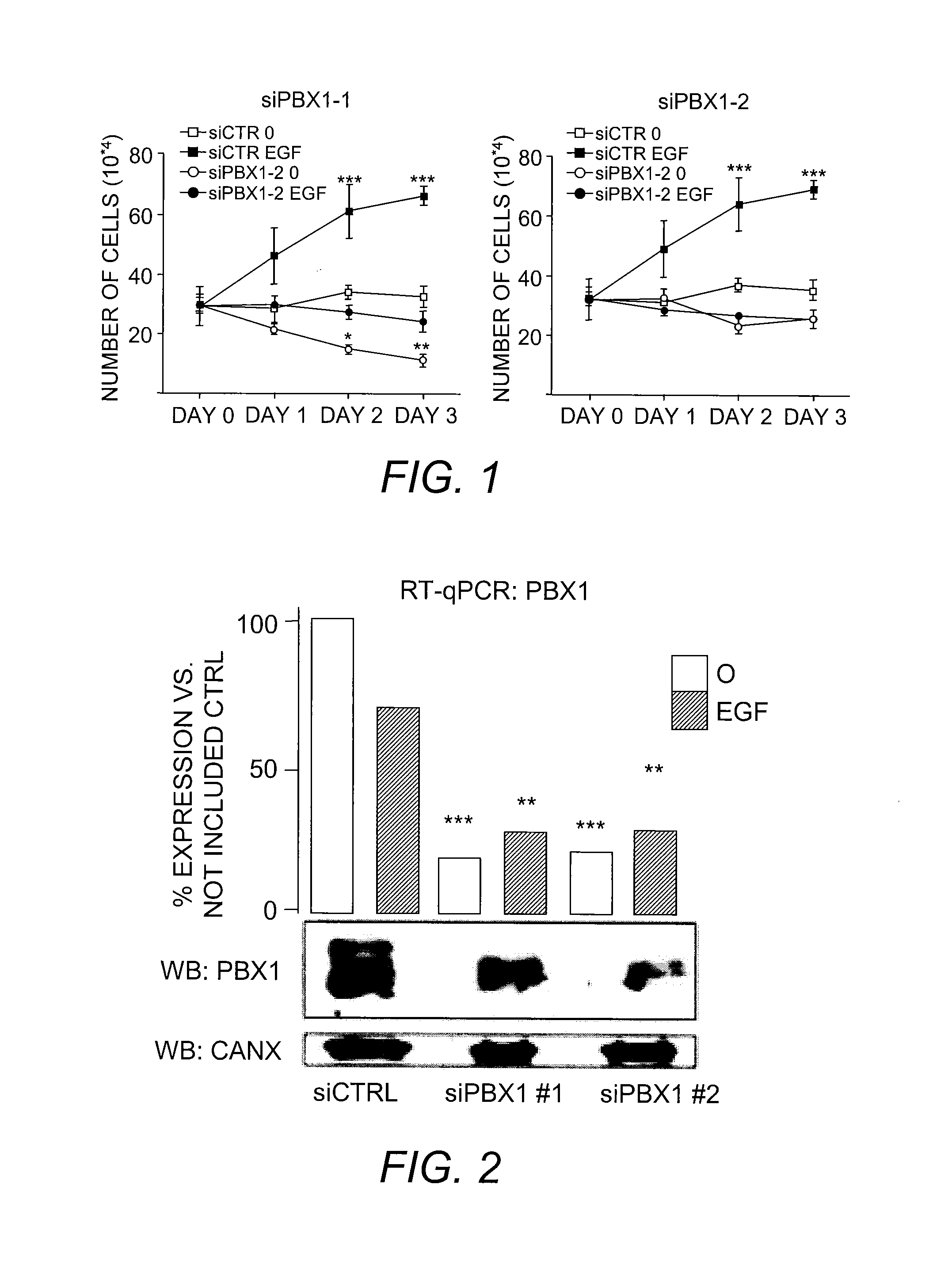
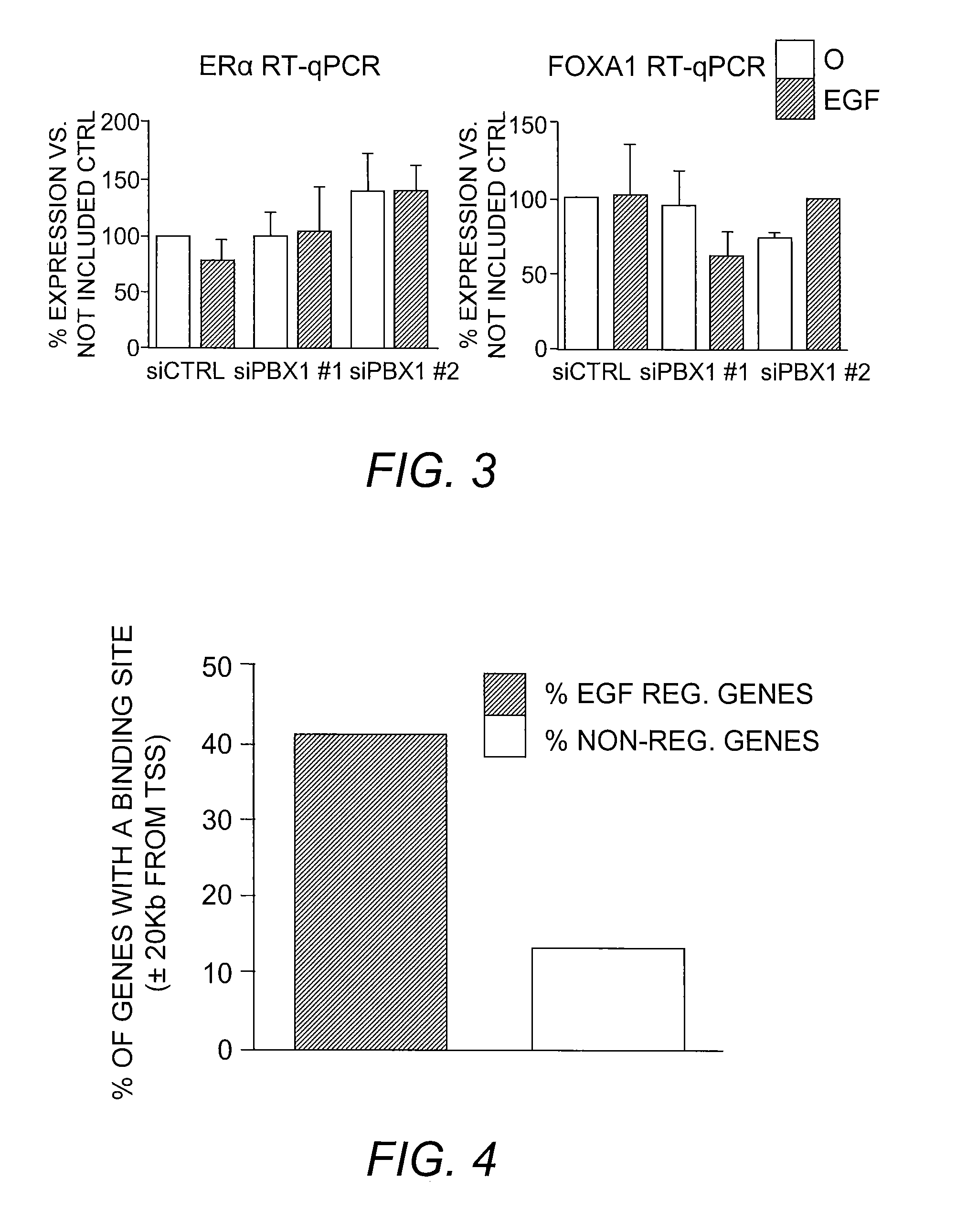
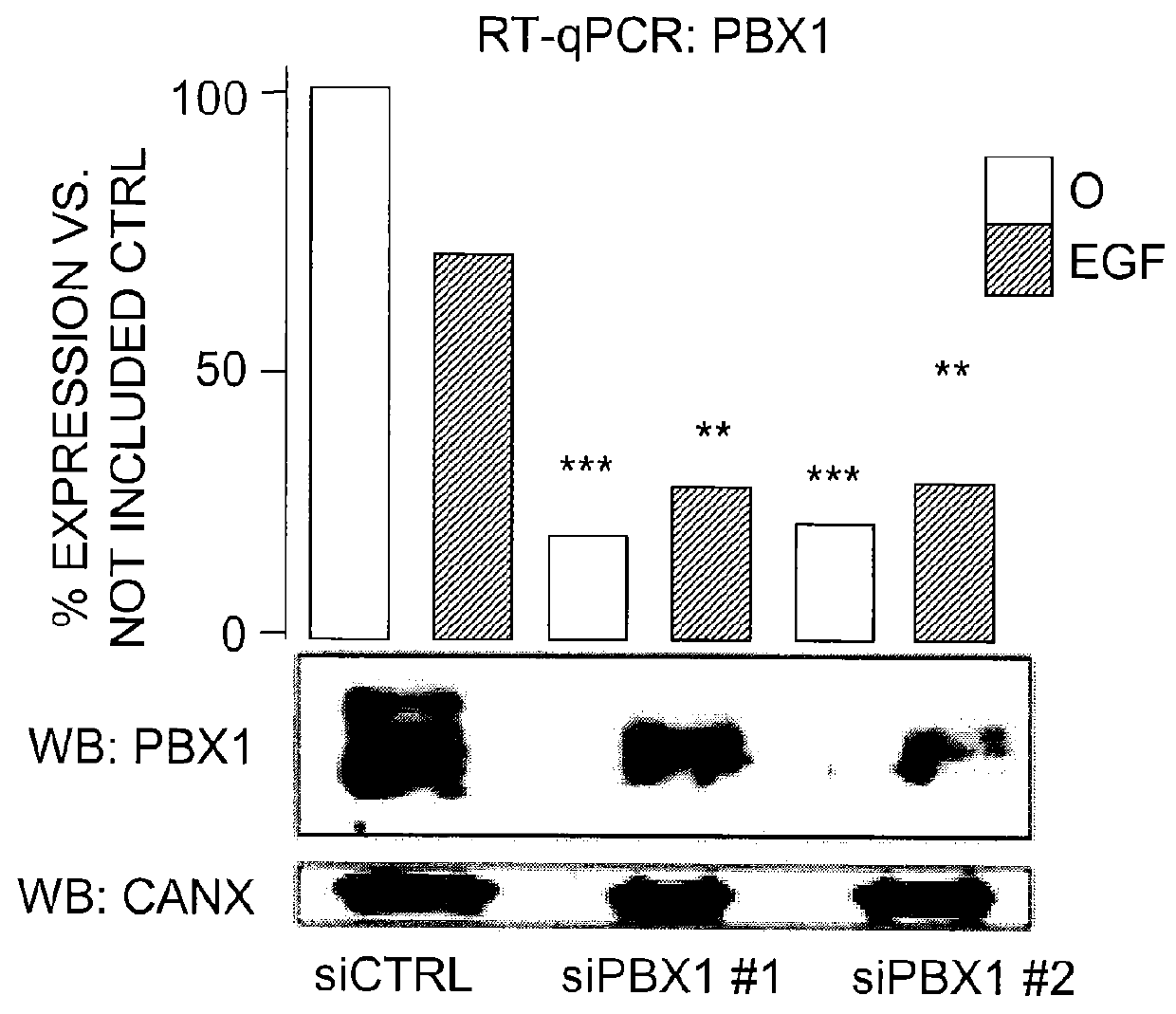
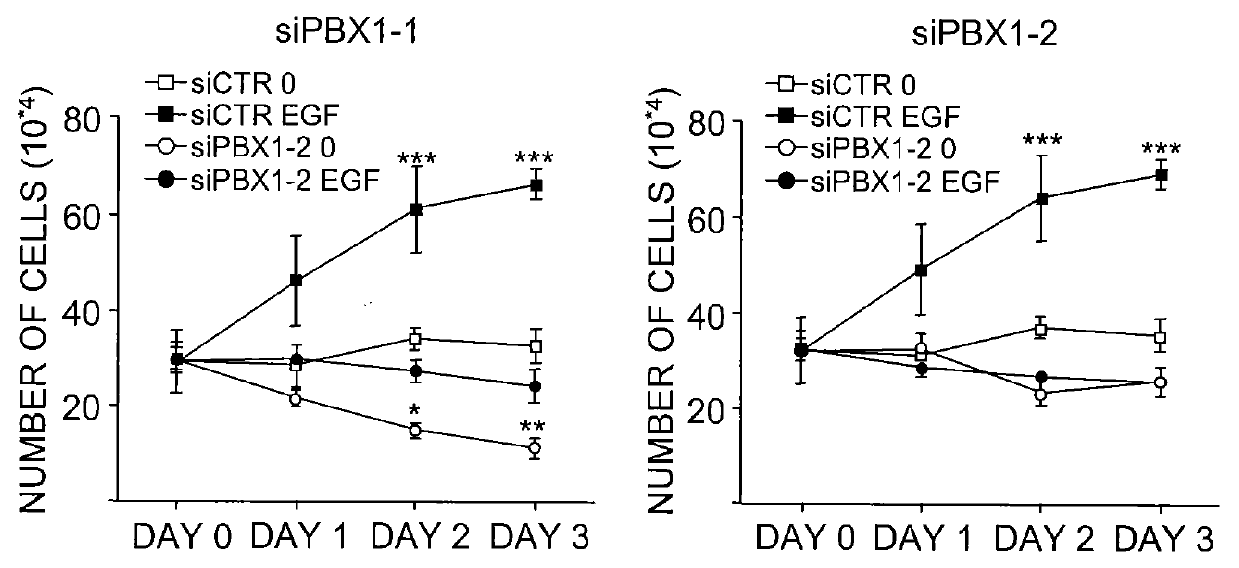
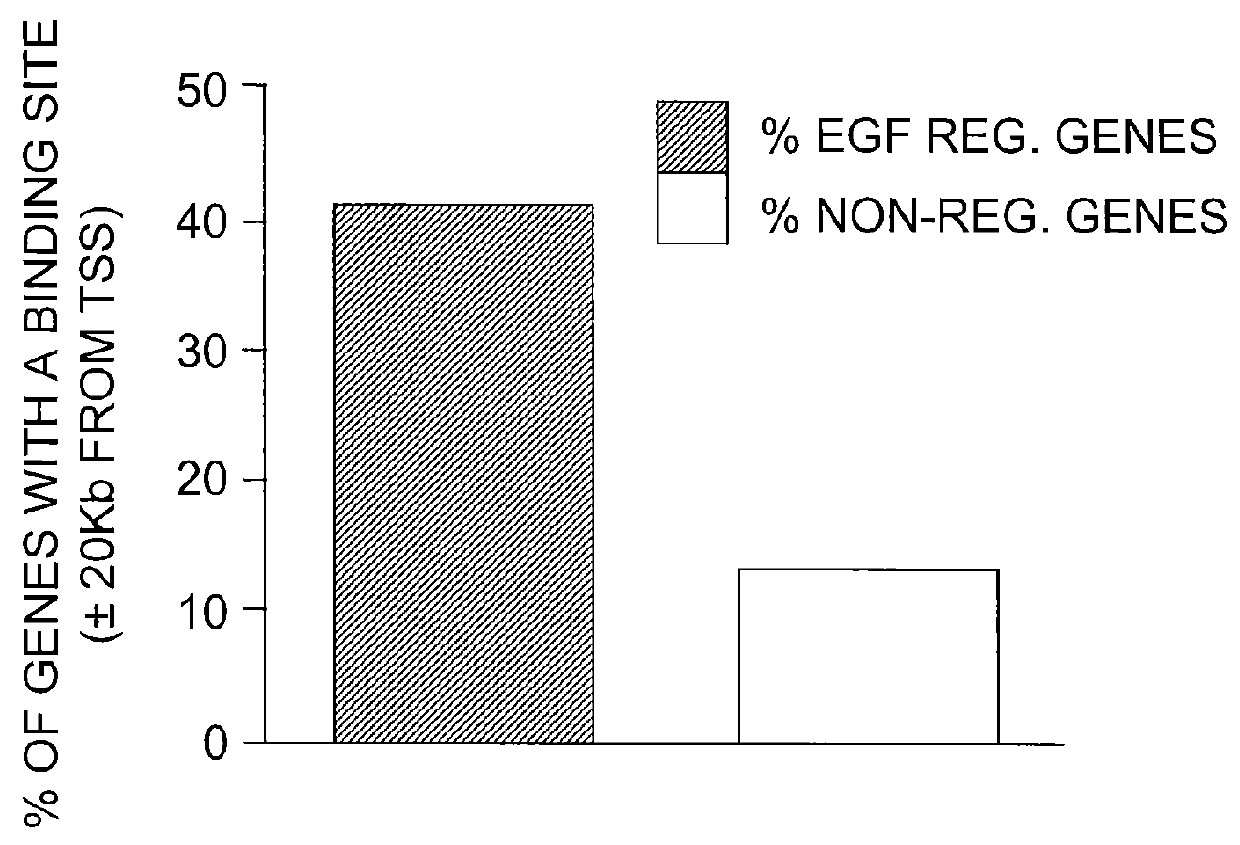
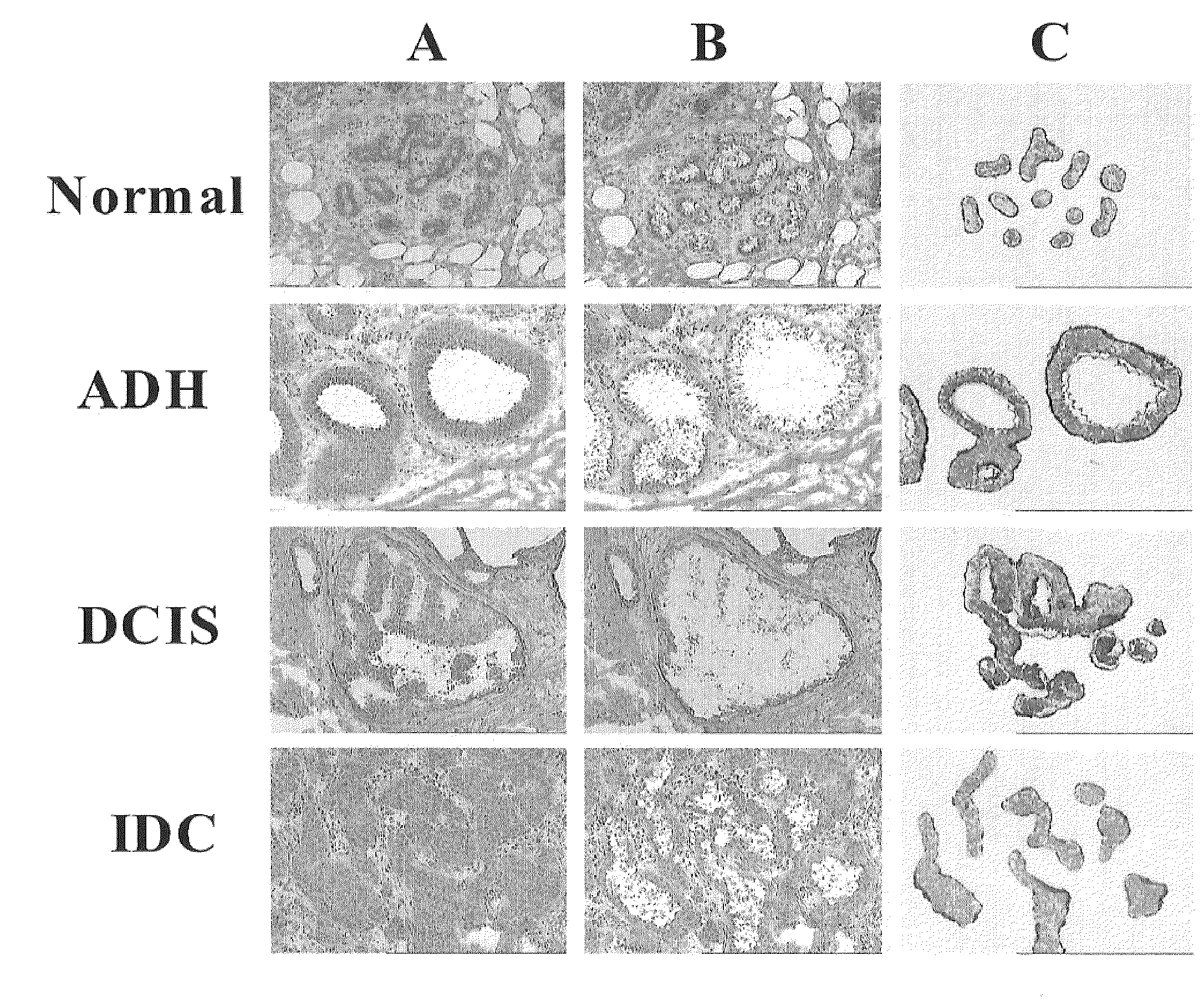
The present invention includes methods for identifying patients who will be resistant to endocrine therapy during breast cancer treatment and determining patient outcome. The methods are based on identifying increased expression of PBX1, or the cistrome signature associated therewith, in breast tissue samples.
Owner:TRUSTEES OF DARTMOUTH COLLEGE THE
Methods for identifying an increased likelihood of recurrence of breast cancer
Methods of identifying a mammal having an increased likelihood of recurrence of breast cancer includes identifying in a breast tissue sample of the mammal expression of at least two genes selected from the group consisting of Hs.125867 (EVL), Hs.591847 (NAT1), Hs.208124 (ESR1), Hs.26225 (GABRP), Hs.408614 (ST8SIA1), Hs.480819 (TBC1D9), Hs.504115 (TRIM29), Hs.523468 (SCUBE2), Hs.532082 (IL6ST), Hs.592121 (RABEP1), Hs.79136 (SLC39A6), Hs.82128 (TPBG), Hs.95243 (TCEAL1), Hs.95612 (DSC2), Hs.654961 (FUT8), Hs.1594 (CENPA), Hs.184339 (MELK), Hs.26010 (PFKP), Hs.592049 (PLK1), Hs.370834 (ATAD2), Hs.437638 (XBP1), Hs.444118 (MCM6), Hs.469649 (BUB1), Hs.470477 (PTP4A2), Hs.473583 (YBX1), Hs.480938 (LRBA), Hs.524134 (GATA3), Hs.531668 (CX3CL1), Hs.532824 (MAPRE2), Hs.591314 (GMPS), Hs.83758 (CKS2) and Hs.99962 (SLC43A3) and subsets of the genes.
Owner:UNIV OF LOUISVILLE RES FOUND INC
Predicting breast cancer treatment outcome
ActiveUS9856533B2ConfidenceSolve the power is smallBioreactor/fermenter combinationsBiological substance pretreatmentsOncologyBiomarker (petroleum)
Methods and compositions are provided for the identification of expression signatures in ER+ breast cancer cases, where the signatures correlate with responsiveness, or lack thereof, to treatment with tamoxifen or another antiestrogen agent against breast cancer The signature profiles are identified based upon sampling of reference breast tissue samples from independent cases of breast cancer and provide a reliable set of molecular criteria for predicting the efficacy of treating a subject with breast cancer with tamoxifen or another antiestrogen agent against breast cancer. Additional methods and compositions are provided for predicting responsiveness to tamoxifen or another antiestrogen agent against breast cancer in cases of breast cancer by use of three biomarkers. Two biomarkers display increased expression correlated with tamoxifen response while the third biomarker displays decreased expression correlated with tamoxifen response.
Owner:THE GENERAL HOSPITAL CORP +1
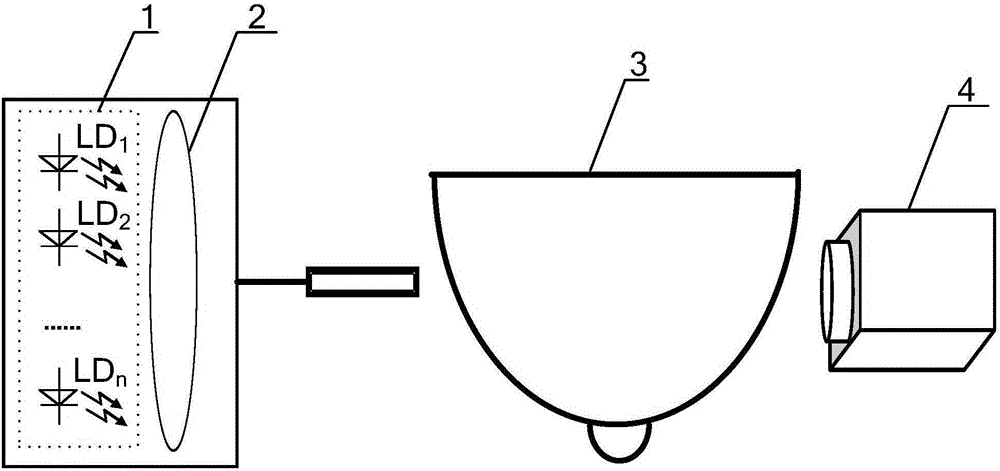
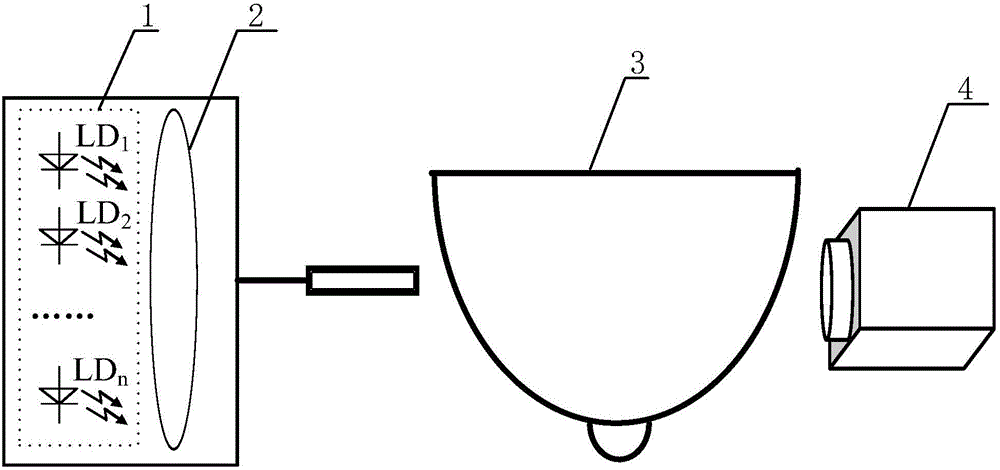
Hyperspectral Imaging measurement system applied on sinusoidal wave frequency coding of breast
ActiveCN105167741AAchieve imagingHigh speedDiagnostic recording/measuringSensorsLight beamOptoelectronics
The invention discloses a hyperspectral imaging measurement system applied on sinusoidal wave frequency coding of a breast. A set of monochromatic light sources are distributed at one side of a breast tissue sample. Cameras are distributed at the other side of the breast tissue sample. The monochromatic light sources in the set of monochromatic light sources are densely arranged on a semi-spherical surface. The light which is emitted from the monochromatic light sources is gathered to a light beam by means of a lens, thereby forming a light source. The cameras form a light receiving device. Sinusoidal waves with different frequencies are used for respectively driving the monochromatic light sources in the set of monochromatic light sources. Each pixel point in an image which is received by the cameras is a combination of monochromatic light that is emitted from the monochromatic light sources and pass through the breast. A computer separates the combination of the monochromatic light for obtaining the contribution of each monochromatic light source in the set of monochromatic light sources, thereby realizing transmission hyperspectral imaging of the breast. The hyperspectral imaging measurement system realizes high-precision measurement for breast transmission hyperspectral imaging with large information amount in high speed. Furthermore the hyperspectral imaging measurement system has advantages of low cost, convenient application, etc. The hyperspectral imaging measurement system is suitable for daily household self measurement.
Owner:TIANJIN UNIV
Method and apparatus for non-compressed evaluation of tissue specimens
InactiveUS20170333892A1Easy to transportEnsure integrityPreparing sample for investigationVaccination/ovulation diagnosticsTissue sampleVisual inspection
The present invention provides a method and apparatus for evaluating the margins of a surgically-removed tissue specimen, such as a breast tissue specimen, to determine whether sufficient fatty tissue has been removed from around the lesion or cancerous point. The instant invention provides a solution to the problem of on-site evaluation of the margin sufficiency during the surgical procedure, in that it provides surgeons with an orthogonal view of all sides of the tissue specimen to be evaluated. The specimen evaluation device provides for properly-oriented examination of the removed specimen in a non-compressed, undistorted manner, both by visual inspection and through radiographic evaluation. Through this examination of the properly-oriented specimen, the surgeon may quickly and more accurately be informed of whether there remain cancerous cells in the margins surrounding the sample, which are meant to be free of cancerous cells. Upon evaluation of the margins of the removed sample, the surgeon may then make an on-site determination on whether to proceed with additional surgery or complete the surgical procedure.
Owner:RUPLEY DANIEL
Automatic glandular and tubule detection in histological grading of breast cancer
Owner:VENTANA MEDICAL SYST INC
Detection and treatment of breast cancer
InactiveUS20140349931A1Efficiently focusImprove the level ofPeptide/protein ingredientsLibrary screeningCancer cellAnti mitotic
The present invention describes methods for determining the risk that a breast precursor lesion will progress to invasive breast cancer and / or the risk of recurrent non-invasive disease in a patient, comprising detecting the presence and / or level of PAPPA and / or PAPPA functional activity in a breast tissue sample obtained from the patient, wherein if PAPPA is not present, or is present at a reduced amount compared to a control, there is the risk of progression to invasive cancer and / or the risk or recurrent disease.The present invention also enables the chemosensitisation of mitotically delayed breast cancer cells to anti-proliferative agents, preferably anti-mitotic agents, by restoring normal progression through mitosis. In this embodiment a first drug is applied to release breast cancer cells from the mitotic block and, sequentially, a second drug affecting proliferating cells is administered for cancer cell killing.
Owner:EURO LAB FUER MOLEKULARBIOLOGIE EMBL +2
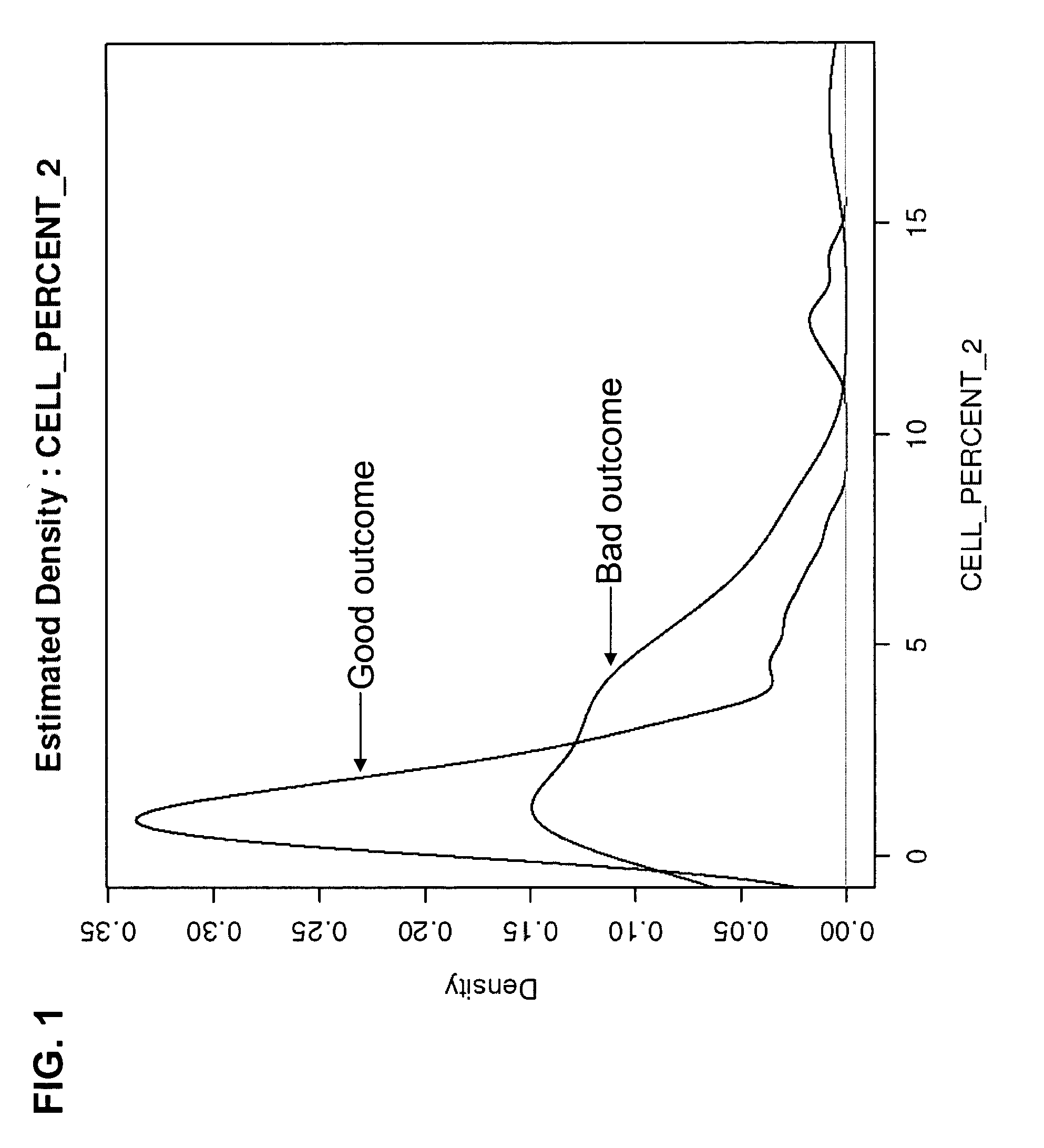
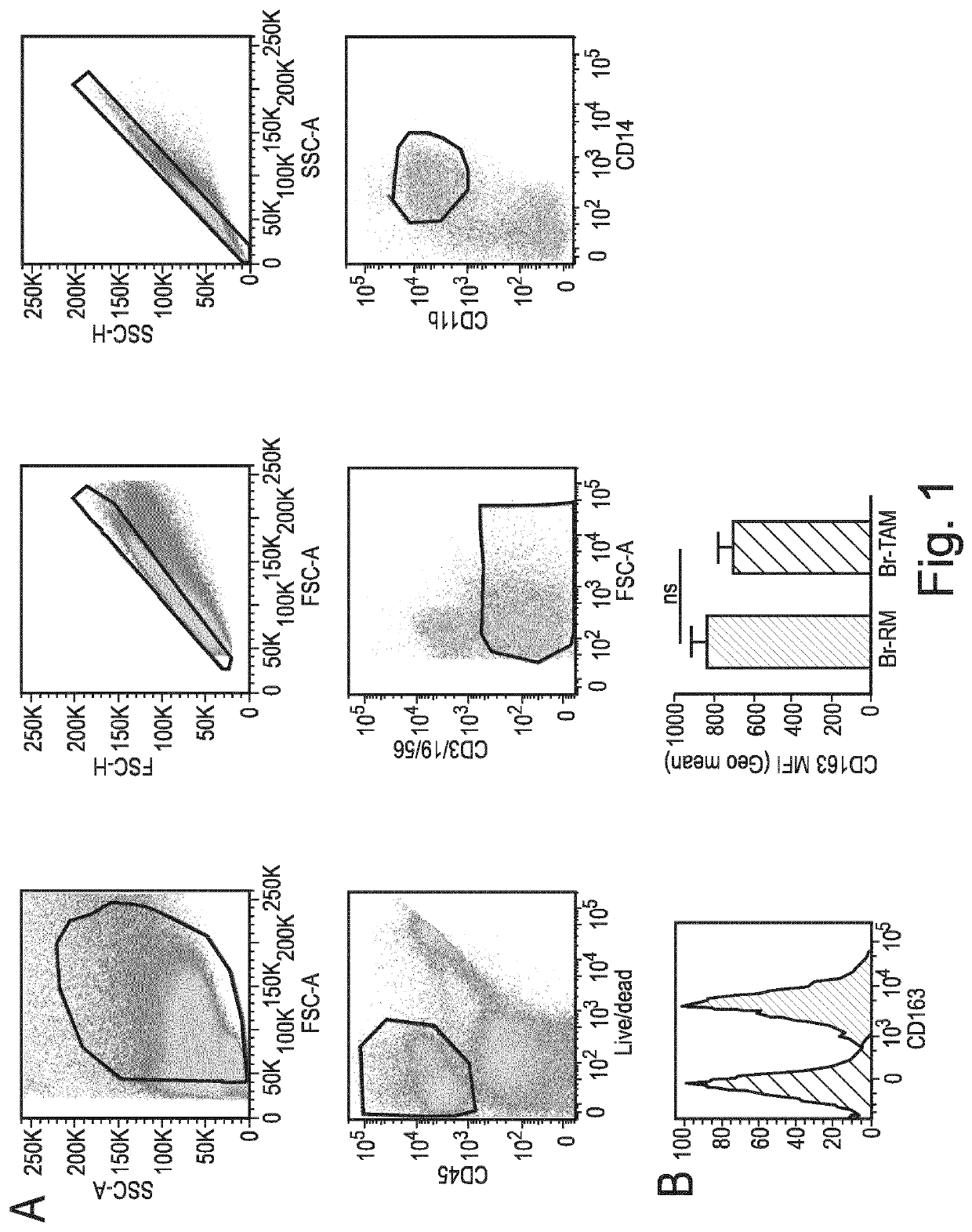
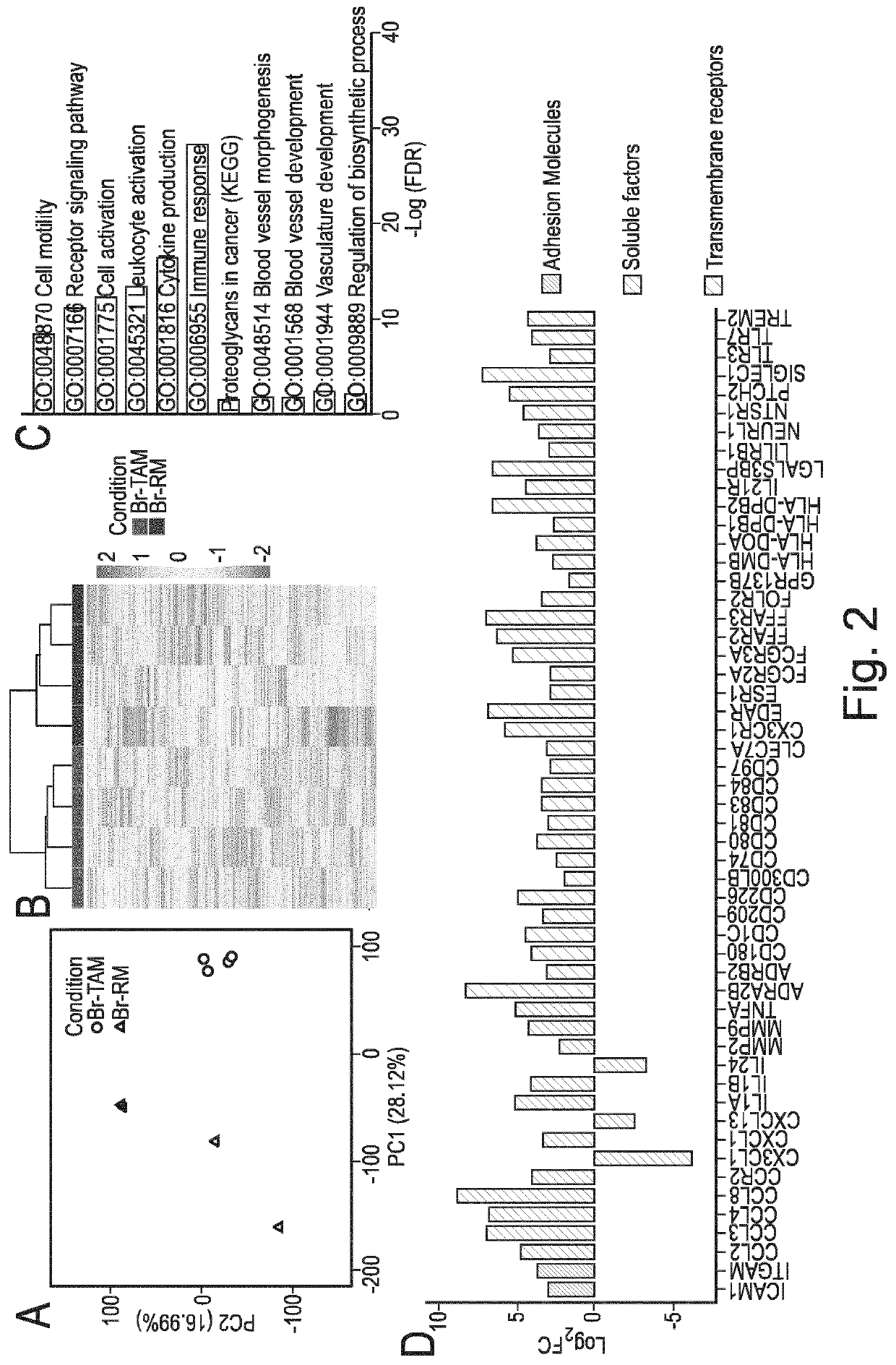
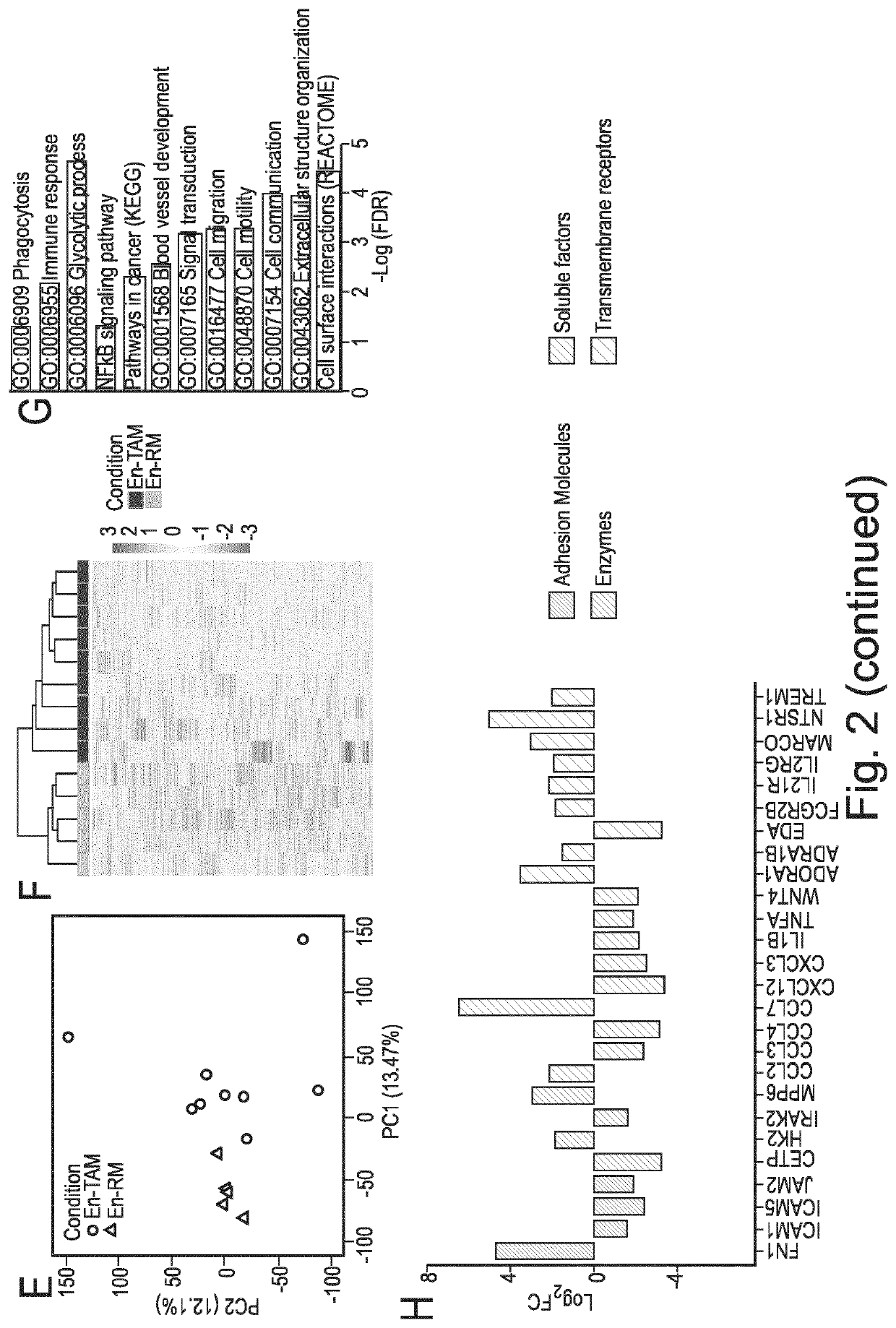
Macrophage expression in breast cancer
PendingUS20220136069A1Address bad outcomesStrong specificityMicrobiological testing/measurementAssayBiologic marker
The present invention relates to the field of breast cancer diagnosis and treatment. The present invention provides methods comprising a) analysing a biological sample obtained from a subject to determine the presence of target molecules representative of expression of at least two biomarkers selected from the group listed in Table 1, wherein the biological sample is a breast tissue sample or derivative thereof; and b) comparing the expression levels of the biomarkers determined in (a) with one or more reference values, wherein whether there is a difference in the expression of the biomarkers in the sample from the subject compared to the one or more reference values is indicative of a clinical indication. The invention further provides methods of treatment and kits and assays for use in the methods of the invention.
Owner:THE UNIV OF EDINBURGH
High spectral imaging system for breast preset electrical level-raised square wave frequency coding
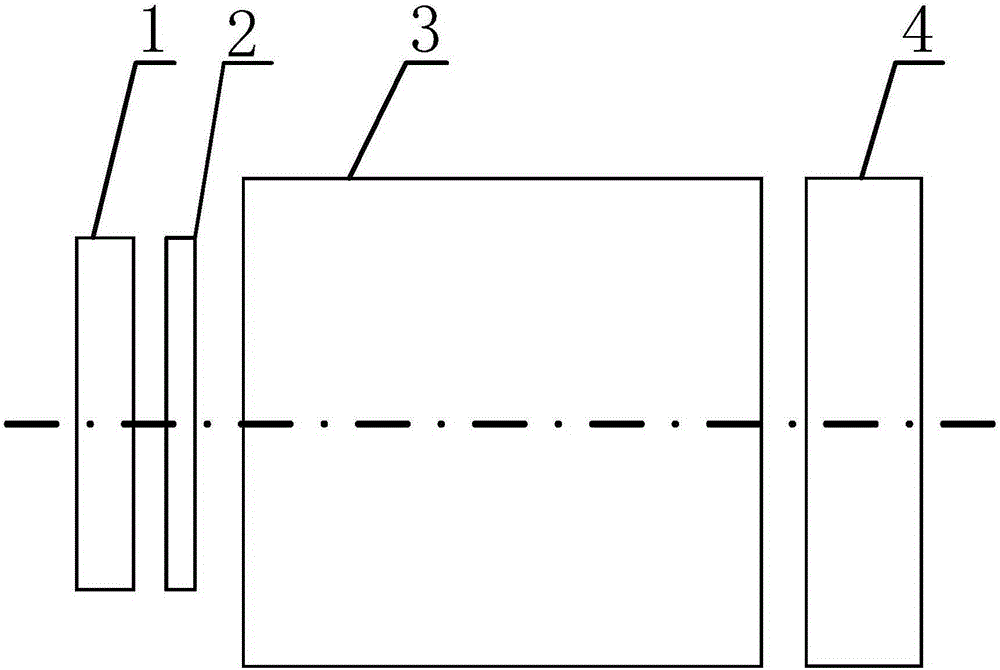
InactiveCN106510621AHigh precisionImprove signal-to-noise ratioDiagnostics using spectroscopySensorsNoise levelSignal-to-quantization-noise ratio
The invention discloses a high spectral imaging system for breast preset electrical level-raised square wave frequency coding. The high spectral imaging system includes driving all the single-color light sources by using preset electrical level-raised square waves with different frequencies, wherein each pixel point of an image received by a camera is a combination of single-color light of each single-color light source penetrating a breast tissue sample. When the camera acquires the image, the noise level does not change, a square signal as a drive is raised in a preset electrical level, the square signal is more obvious in improvement than noise in a low electrical level part of the square signal, the signal to noise ratio of the image acquired by the camera is improved in a low electrical level segment of the square signal, and then the precision of the combination of the single-color light input in a computer can be improved; and the computer can separate the combination of the single-color light so as to acquire a contribution of each single-color light source in the combination of the single-color light, and accordingly, imaging of transmission high spectrogram of the breast tissue sample can be achieved.
Owner:TIANJIN UNIV
Method and apparatus for non-compressed evaluation of tissue specimens
InactiveUS10016760B2Easy to transportEnsure integrityPreparing sample for investigationVaccination/ovulation diagnosticsTissue sampleVisual inspection
The present invention provides a method and apparatus for evaluating the margins of a surgically-removed tissue specimen, such as a breast tissue specimen, to determine whether sufficient fatty tissue has been removed from around the lesion or cancerous point. The instant invention provides a solution to the problem of on-site evaluation of the margin sufficiency during the surgical procedure, in that it provides surgeons with an orthogonal view of all sides of the tissue specimen to be evaluated. The specimen evaluation device provides for properly-oriented examination of the removed specimen in a non-compressed, undistorted manner, both by visual inspection and through radiographic evaluation. Through this examination of the properly-oriented specimen, the surgeon may quickly and more accurately be informed of whether there remain cancerous cells in the margins surrounding the sample, which are meant to be free of cancerous cells. Upon evaluation of the margins of the removed sample, the surgeon may then make an on-site determination on whether to proceed with additional surgery or complete the surgical procedure.
Owner:RUPLEY DANIEL
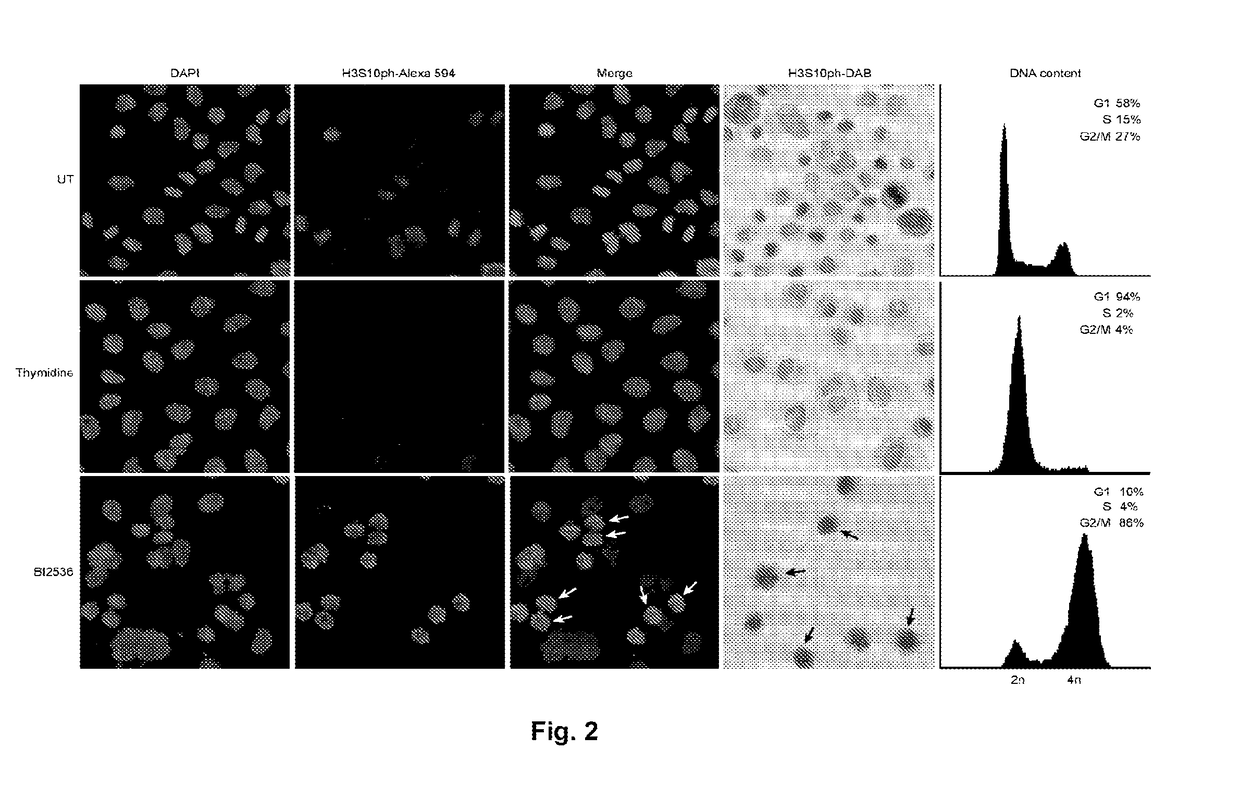
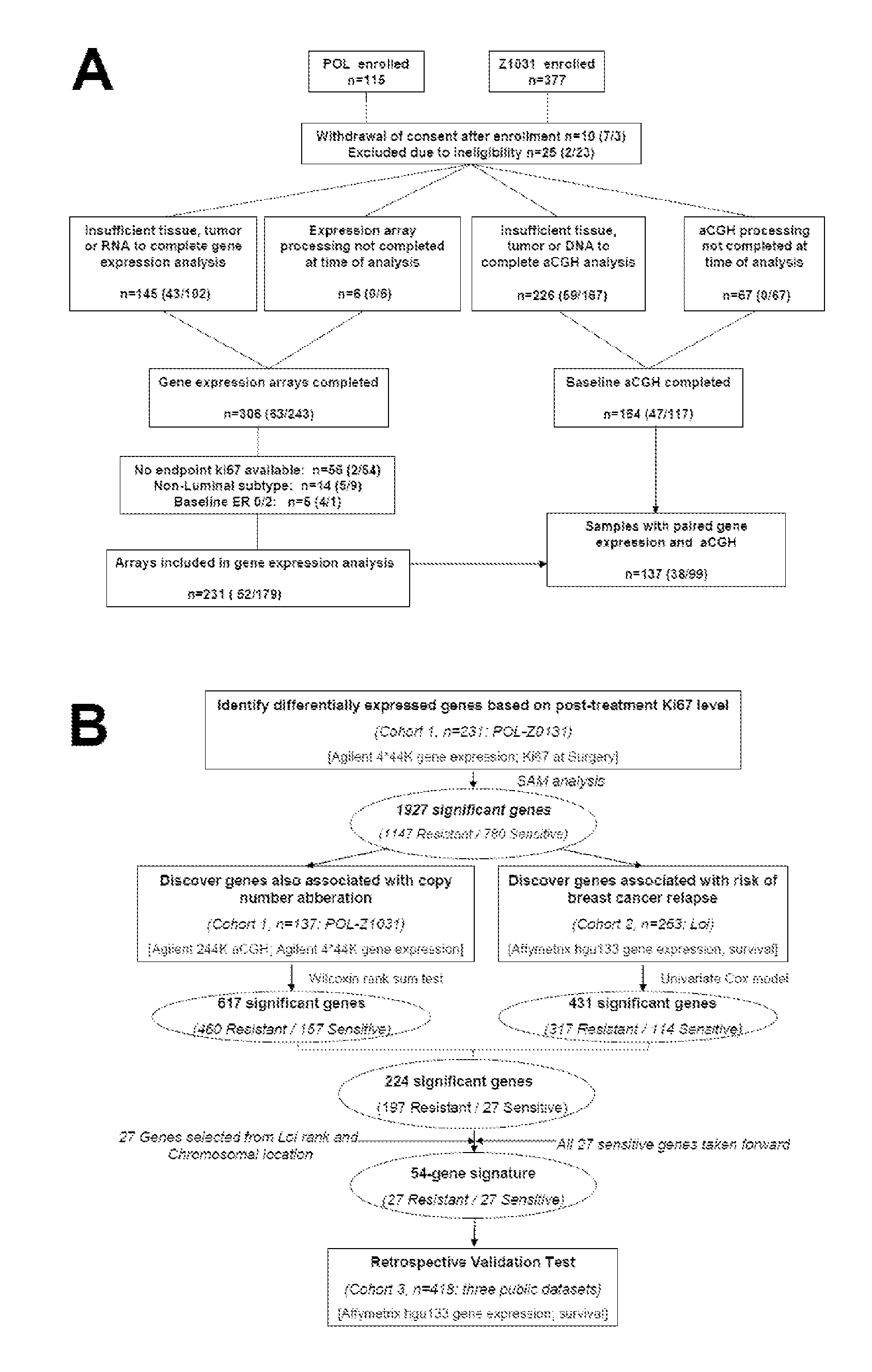
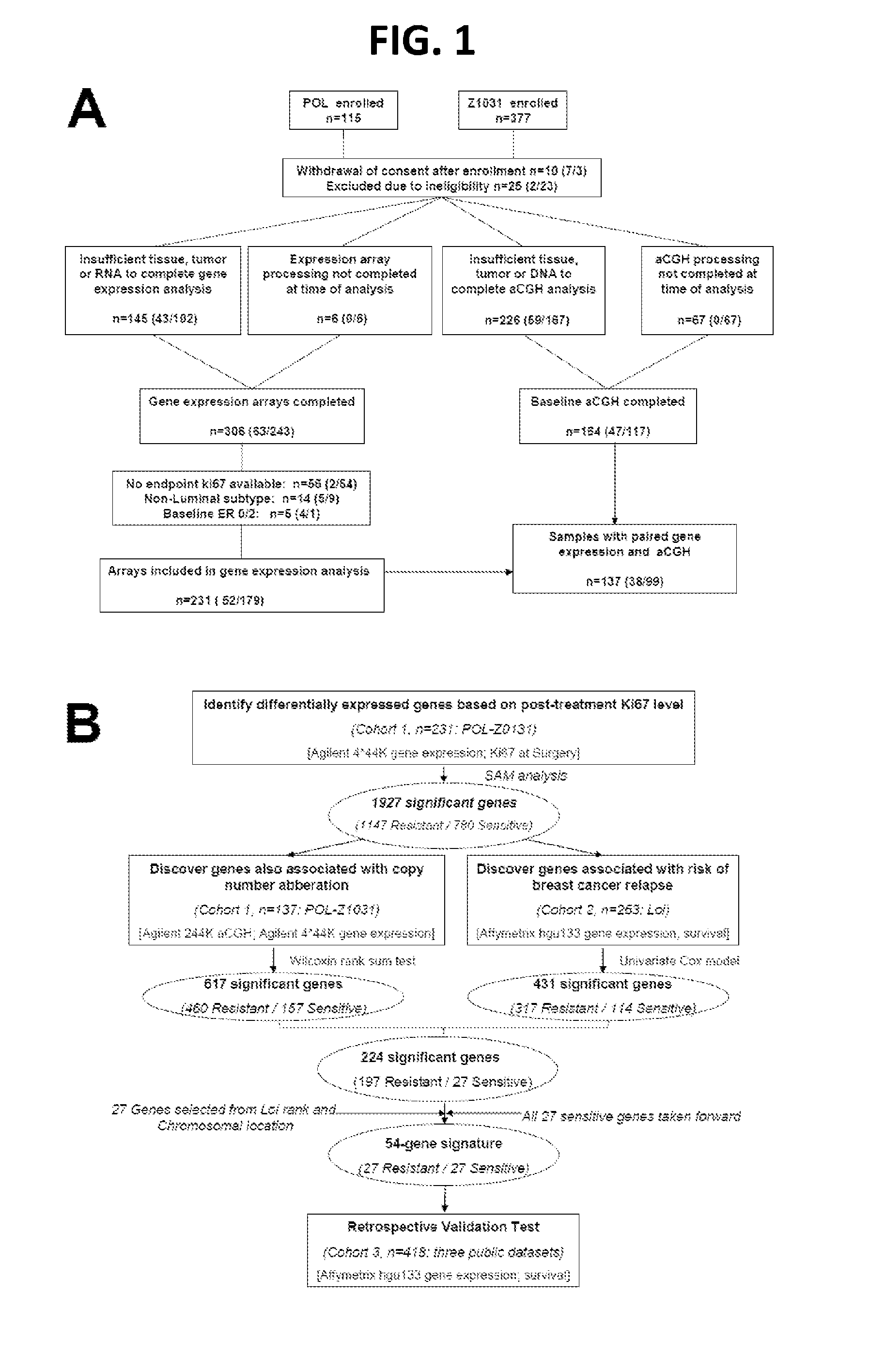
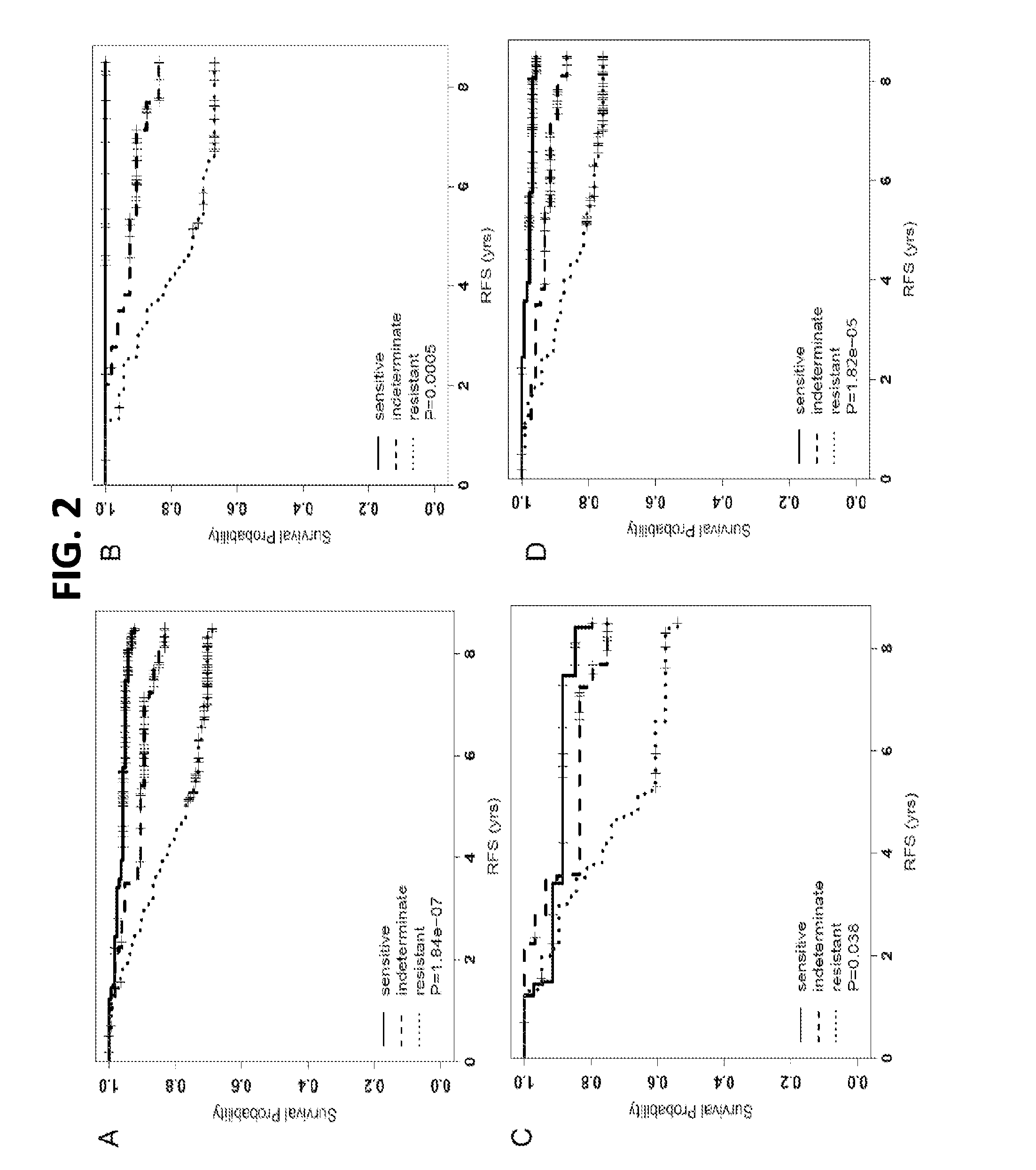
Copy number aberration driven endocrine response gene signature
InactiveUS9890430B2Easy to optimizeImprove performanceSequential/parallel process reactionsMicrobiological testing/measurementAdjuvantBreast tissue sample
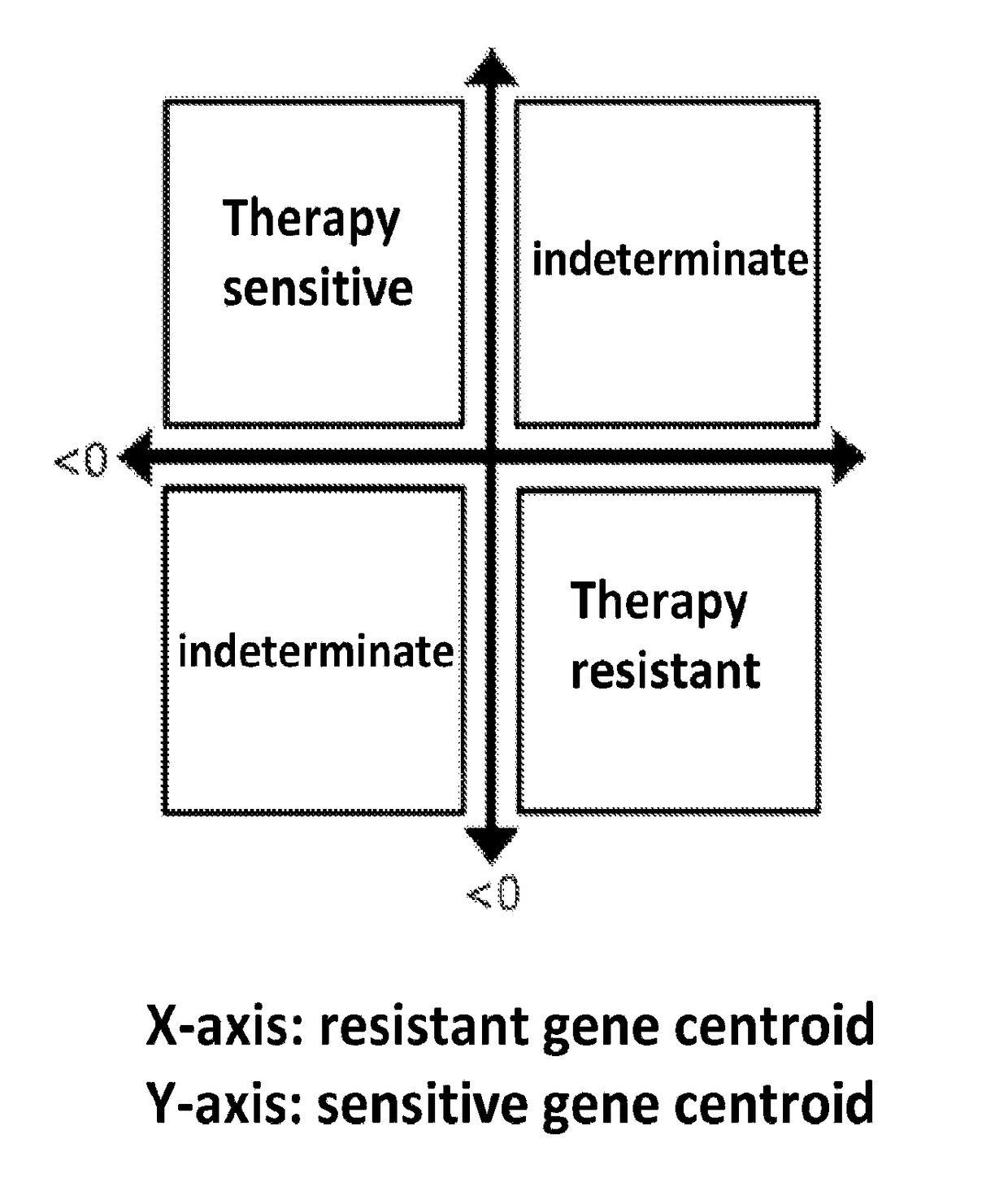
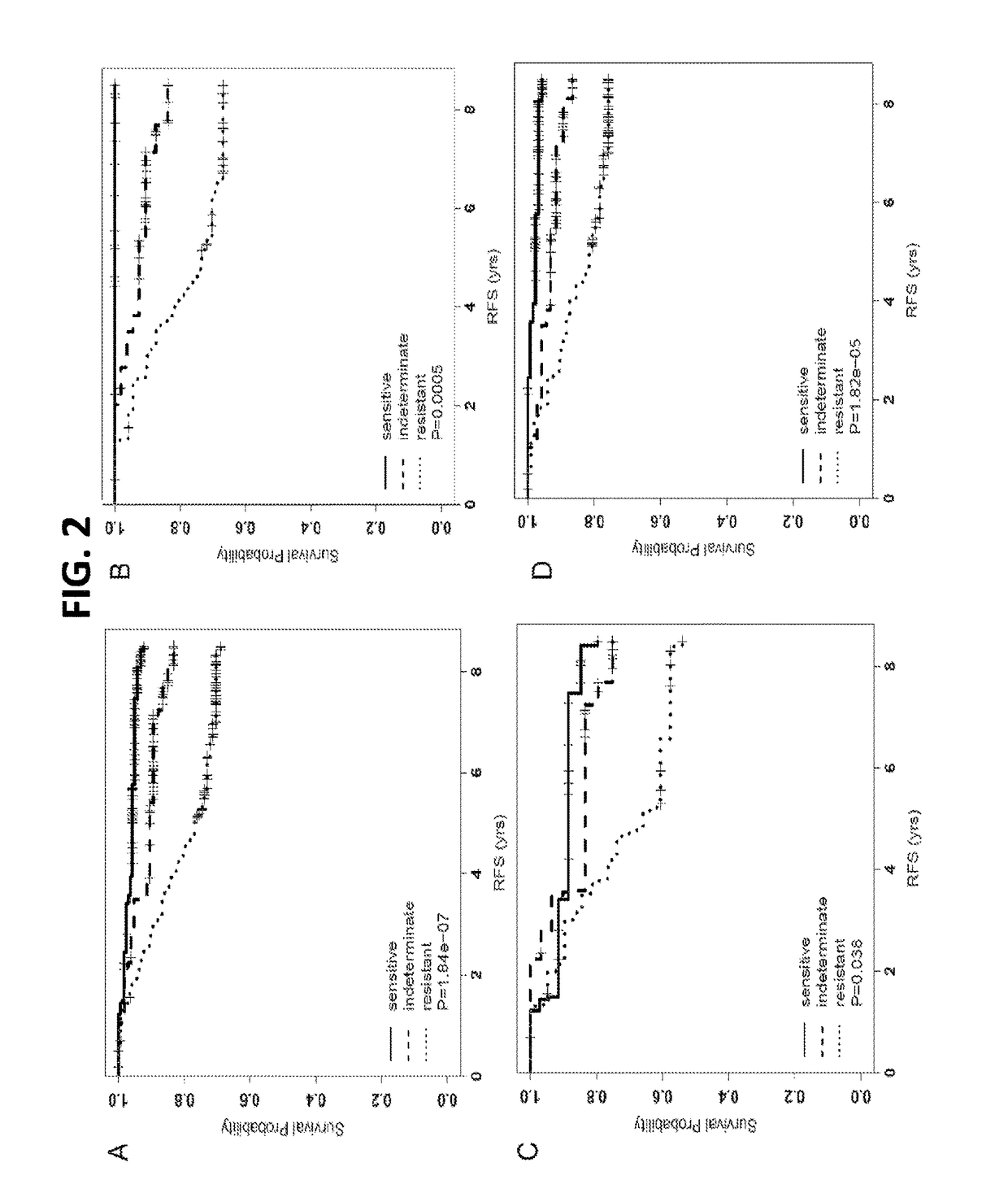
Disclosed are methods of predicting the likelihood of long-term survival without recurrence of breast cancer for a subject having estrogen receptor-positive (ER+) breast cancer treated with adjuvant endocrine monotherapy. In various embodiments, these methods comprise performing a gene expression profile of a breast tissue sample of substantially all of the genes of the “CADER set” described herein; calculating a risk score using a regression model; and applying a double median cutoff classification to assign the subject to a sensitive, indeterminate or resistant group, wherein assignment to a sensitive group predicts longer relapse-free survival compared to the median relapse-free survival of ER+ breast cancer patients treated with adjuvant endocrine monotherapy.
Owner:WASHINGTON UNIV IN SAINT LOUIS
Methods for identifying an increased likelihood of recurrence of breast cancer
Owner:UNIV OF LOUISVILLE RES FOUND INC
Methods for evaluating breast cancer prognosis
InactiveUS20150344962A1Accurate assessmentImproved prognosisNucleotide librariesMicrobiological testing/measurementCancer cellBiologic marker
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Detection and treatment of breast cancer
InactiveUS20170298438A1Efficiently focusImprove the level ofPeptide/protein ingredientsMicrobiological testing/measurementCancer cellFunctional activity
The present invention describes methods for determining the risk that a breast precursor lesion will progress to invasive breast cancer and / or the risk of recurrent non-invasive disease in a patient, comprising detecting the presence and / or level of PAPA and / or PAPPA functional activity in a breast tissue sample obtained from the patient, wherein if PAPPA is not present, or is present at a reduced amount compared to a control, the is the risk of progression to invasive cancer and / or the risk or recurrent disease.The present invention also enables the chemosensitisation of mitotically delayed breast cancer cells to anti-proliferative agents, preferably anti-mitotic agents, by restoring normal progression through mitosis. In this embodiment a first drug is applied to release breast cancer cells from the mitotic block and, sequentially, a second drug affecting proliferating cells is administered for cancer cell killing.
Owner:EURO LAB FUER MOLEKULARBIOLOGIE EMBL +2
Copy number aberration driven endocrine response gene signature
InactiveUS20150240312A1Better assay performanceEasy to optimizeSequential/parallel process reactionsMicrobiological testing/measurementAdjuvantIntracrine
Disclosed are methods of predicting the likelihood of long-term survival without recurrence of breast cancer for a subject having estrogen receptor-positive (ER+) breast cancer treated with adjuvant endocrine monotherapy. In various embodiments, these methods comprise performing a gene expression profile of a breast tissue sample of substantially all of the genes of the “CADER set” described herein; calculating a risk score using a regression model; and applying a double median cutoff classification to assign the subject to a sensitive, indeterminate or resistant group, wherein assignment to a sensitive group predicts longer relapse-free survival compared to the median relapse-free survival of ER+ breast cancer patients treated with adjuvant endocrine monotherapy.
Owner:WASHINGTON UNIV IN SAINT LOUIS
Markers for identifying breast cancer treatment modalities
InactiveUS9382588B2Microbiological testing/measurementImmunoglobulins against cell receptors/antigens/surface-determinantsEndocrine therapyOncology
The present invention includes methods for identifying patients who will be resistant to endocrine therapy during breast cancer treatment and determining patient outcome. The methods are based on identifying increased expression of PBX1, or the cistrome signature associated therewith, in breast tissue samples.
Owner:TRUSTEES OF DARTMOUTH COLLEGE THE
Grading of breast cancer
InactiveUS20110183858A1Accurate assessmentDetermining prognosisOrganic active ingredientsMicrobiological testing/measurementEarly breast cancerOncology
Methods and compositions for the identification of breast cancer grade signatures are provided. The signature profiles are identified based upon multiple sampling of reference breast tissue samples from independent cases of breast cancer and provide a reliable set of molecular criteria for identification of cells as being in one or more particular stages and / or grades of breast cancer.
Owner:THE GENERAL HOSPITAL CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com