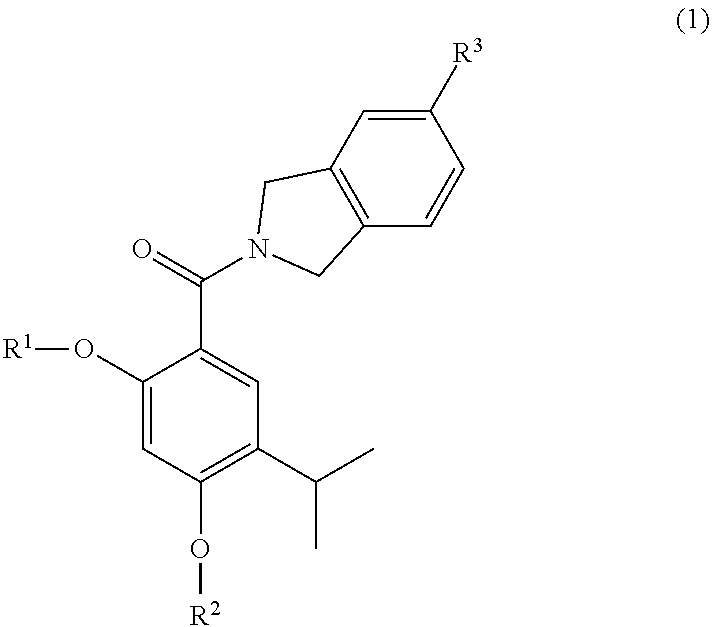
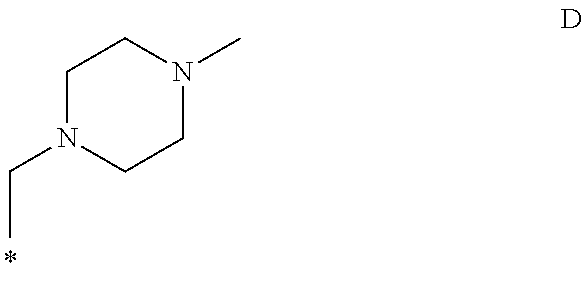
Pharmaceutical compounds
a technology of compound and compound, applied in the field of compound compound, can solve the problems of increasing the production of non-native or mutant proteins, increasing the requirement for chaperone systems, and g2/m arrest generally less well tolerated by cells, so as to prevent any pain from developing, reduce or even eliminate pain, and prevent existing pain from worsening
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0687]The invention will now be illustrated, but not limited, by reference to the specific embodiments described in the following examples.
[0688]In the examples, the following abbreviations may be used.[0689]AcOH acetic acid[0690]BOC tert-butyloxycarbonyl[0691]Bn benzyl[0692]CDI 1,1-carbonyldiimidazole[0693]DMAW90 Solvent mixture: DCM: MeOH, AcOH, H2O (90:18:3:2)[0694]DMAW120 Solvent mixture: DCM: MeOH, AcOH, H2O (120:18:3:2)[0695]DMAW240 Solvent mixture: DCM: MeOH, AcOH, H2O (240:20:3:2)[0696]DCM dichloromethane[0697]DMF dimethylformamide[0698]DMSO dimethyl sulphoxide[0699]EDC 1-ethyl-3-(3′-dimethylaminopropyl)-carbodiimide[0700]Et3N triethylamine[0701]EtOAc ethyl acetate[0702]Et2O diethyl ether[0703]h hour(s)[0704]HOAt 1-hydroxyazabenzotriazole[0705]HOBt 1-hydroxybenzotriazole[0706]MeCN acetonitrile[0707]MeOH methanol[0708]min. minutes[0709]P.E. petroleum ether[0710]r.t. room temperature[0711]SiO2 silica[0712]TBTU N,N,N′,N′-tetramethyl-O-(benzotriazol-1-yl)uronium tetrafluoroborat...
preparation a2
Methyl 2,4-dihydroxy-5-isopropylbenzoate
[0733]
[0734]10% Palladium on carbon (350 mg) was added to a suspension of methyl 2,4-bis-benzyloxy-5-isopropenylbenzoate [prepared as per WO 2006 / 109085 A1] (3.88 g, 10.0 mmol) in ethanol (30 ml) and the mixture was stirred at room temperature under a hydrogen atmosphere for 1 hour. Methanol (20 ml) was added to aid dissolution and the mixture was stirred at room temperature under a hydrogen atmosphere for 16 hours. The mixture was filtered, the catalyst was rinsed with methanol (3×20 ml) and the combined filtrates were evaporated in vacuo to afford methyl 2,4-dihydroxy-5-isopropylbenzoate (2.10 g, 100%) as a colourless solid. 1H NMR (DMSO-d6) 10.54 (1H, s), 10.44 (1H, br s), 7.52 (1H, s), 6.37 (1H, s), 3.85 (3H, s), 3.08 (1H, m), 1.13 (6H, d). MS: [M+H]+ 211.
Preparation A3
4-Hydroxy-5-isopropyl-2-methoxybenzoic acid
[0735]
[0736]A mixture of methyl 2,4-dihydroxy-5-isopropylbenzoate (1.05 g, 5.0 mmol) and anhydrous potassium carbonate (828 mg, 6....
preparation a10
2-Hydroxy-5-isopropyl-4-(methoxymethyloxy)benzoic acid
[0761]
[0762]Aqueous potassium hydroxide (50% w / v, 1 ml) was added to a mixture of methyl 2-hydroxy-5-isopropyl-4-(methoxymethyloxy)benzoate (508 mg, 2.0 mmol) in methanol (10 ml) and water (4 ml) and the mixture was stirred and held at reflux for 6 hours. Upon cooling to room temperature the organic solvent was removed in vacuo and the residue acidified by the addition of 2M hydrochloric acid (30 ml). The solid material was collected by suction filtration, rinsed with water (2×20 ml) and sucked dry under reduced pressure to afford 2-hydroxy-5-isopropyl-4-(methoxymethyloxy)benzoic acid (400 mg, 83%) as a colourless solid. 1H NMR (DMSO-d6) 13.60 (1H, br s), 11.30 (1H, br s), 7.58 (1H, s), 6.58 (1H, s), 5.30 (2H, s), 3.42 (3H, s), 3.15 (1H, m), 1.18 (6H, d). MS: [M+H]+ 241.
Preparation A11
2,4-Bis-(methoxymethyloxy)-5-isopropylbenzoic acid
[0763]
[0764]A mixture of methyl 2,4-dihydroxy-5-isopropylbenzoate (420 mg, 2.0 mmol) and anhydrou...
PUM
| Property | Measurement | Unit |
|---|---|---|
| therapeutic time | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com