Microbially expressed xylanases and their use as feed additives and other uses
a technology of xylanases and coding sequences, which is applied in the field of codonoptimized xylanase coding sequences and the expression of xylanases, can solve the problems of xylanase design for feed applications that are not very thermotolerant, xylanase application is not as simple, and xylanase conversion ratios that are used in feed applications are not easy to achieve. , to achiev
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
XylA1A Expression Constructs
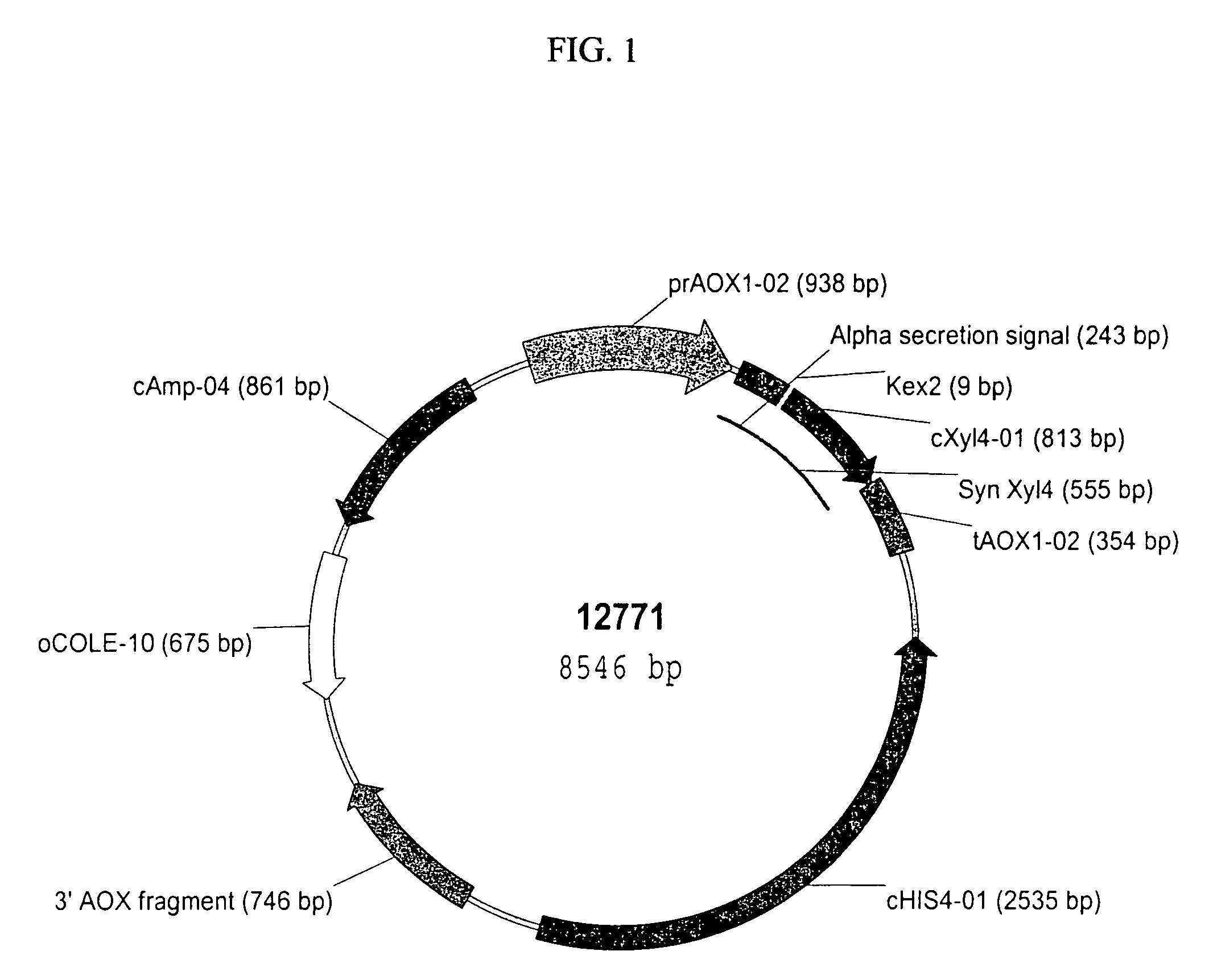
[0157]pBSC12771 (pPIC9 harboring the Synthetic XylA1A Xylanse Gene). A synthetic gene encoding the XylA1A xylanase amino acid sequence was constructed at Entelechon (Regensburg, Germany) utilizing Pichia pastoris preferred codons and was designated PP6002 (for Pichia pastoris optimized codon version of XylA1A) (SEQ ID NO:1). The synthetic gene sequence was designed to include the KEX2 protease cleavage signal (Glu-Lys-Arg) (SEQ ID NO: 37 and nucleotide sequence SEQ ID NO:36) in front of the mature peptide coding sequence. The synthetic gene was supplied in the pPIC9 vector (Invitrogen, Carlsbad, Calif.) and was designated pBSC12771 by Syngenta Quality Control (FIG. 1 and SEQ ID NO:33). This cloning strategy produced a fusion protein in which the Saccharomyces cerevisiae α-mating factor pre-pro-peptide secretion signal (nucleotide and amino acid sequences, SEQ ID NOS: 38 and 39, respectively) is fused in frame to the N-terminus of the PP6002 gene sequence....
example 2
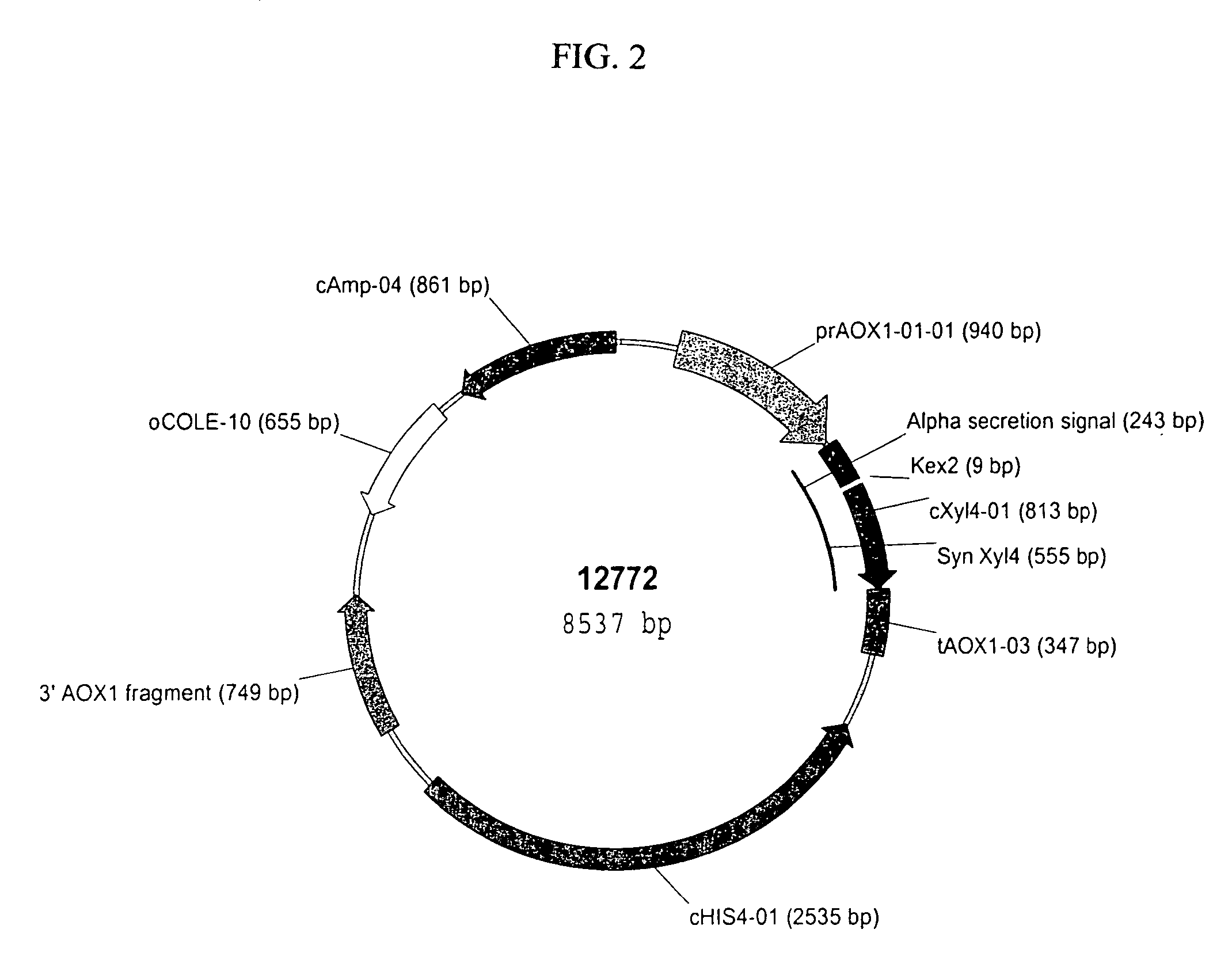
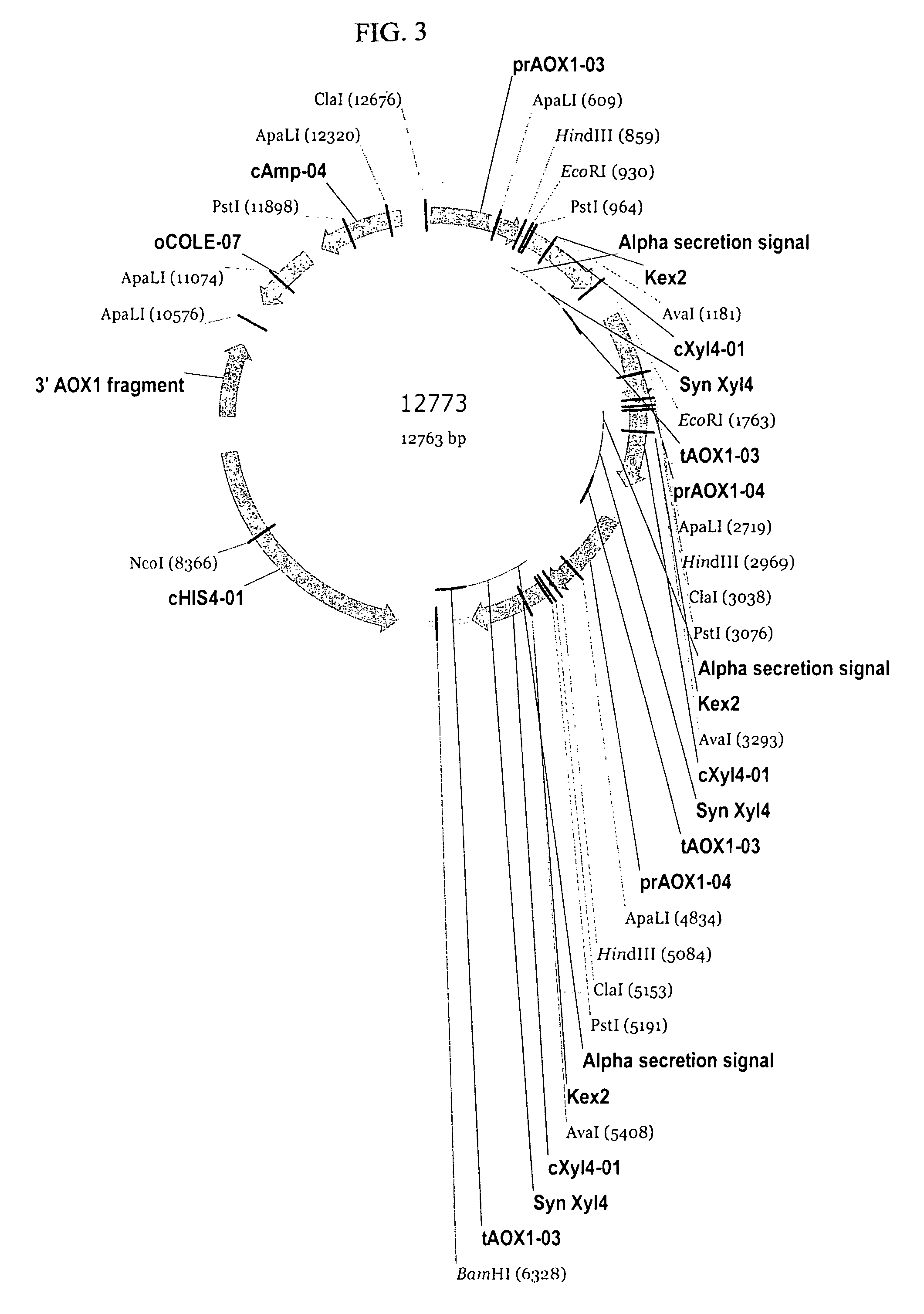
[0171]Preparation of pSYN12773 DNA for Transformation of P. pastoris. A 50 mL culture of TB broth supplemented with ampicillin (100 μg / mL) was inoculated with the glycerol stock of E. coli TOP10 cells harboring pSYN12773, and grown over-night at 37° C. DNA was purified from the culture by methods described by Qiagen (Qiaprep Midiprep protocol, Qiagen, Valencia, Calif.). The isolated plasmid DNA was digested over-night with BglII endonuclease (New England Biolabs, Beverly, Mass.). The digestion mix was electrophoresed through a 0.8% Tris Acetate EDTA (TAE) agarose gel and the 10.4 kb fragment corresponding to the XylA1A integration cassette purified from the gel by methods described by Qiagen (QiaQuick gel purification protocol, Valencia, Calif.). A portion of the purified fragment was electrophoresed through a 0.8% TAE gel to confirm complete digestion and its relative concentration. In addition, a portion of the purified fragment was transformed into chemically competent E. coli DH...
example 3
Construction of P. pastoris XylA1A Expression Host
[0172]Preparation of P. pastoris GS115 Cells for Transformation. All microbiological manipulations were conducted in a laminar flow hood using aseptic techniques. Pichia pastoris GS115 yeast cells (Invitrogen, Carlsbad, Calif.) were prepared by streaking the cells onto YPD agarose plates. Following overnight growth at 30° C., a single yeast colony from the YPD agarose plate was transferred to 5 ml of YPD broth and grown at 30° C. overnight. A portion of this “seed culture” was used to inoculate a sterile 2-liter, baffeled flask containing 500 mL of YPD broth. This culture was grown with vigorous shaking overnight at 30° C. to an optical density OD600=1.5. The cells were harvested by centrifugation at 4000×g, 4° C., 5 minutes, and resuspended in 80 mL of sterile double distilled (sdd) water. Ten milliliters of 10×TE buffer (10 mM Tris-HCl, 0.1 mM EDTA), pH 7.5 was added to the suspension followed by 10 mL of 1M lithium acetate (LiAc)....
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com