Method of reclaiming brine solutions using an organic chelant
a technology of organic chelating agent and brine solution, which is applied in the direction of sealing/packing, separation process, and borehole/well accessories, etc., can solve the problems of affecting the acceptance of brine solutions, fluids may or may not have further practical or economic value, and the cost of initial purchase and subsequent disposal of brines, so as to achieve a lower solubility valence state
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
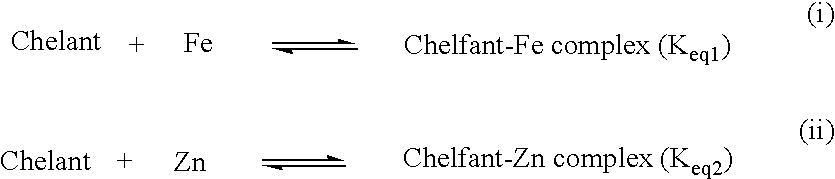
Examples
example 1
[0037]2.03 grams of LED3A slurry, which is 20.9% LED3A in 14.2 ppg CaBr2, were added to 350 ml of 17.4 ppg brine. The initial iron content of the brine was 150 mg / L (72 ppm). The brine was stirred for 8 hours after which the stirrer was turned off. After a further 47 hours a sample was taken and filtered. The iron content was then found to be 35 mg / L (17 ppm).
example 2
[0038]0.082 grams of LED3A (0.4 ppb) were added to 70 ml of 16.9 ppg brine. The initial iron content was 512 mg / L (253 ppm). The brine solution was mixed for 15 hours. After which a sample was taken and filtered. The iron content was subsequently measured at 42 mg / L (21 ppm).
example 3
[0039]12 grams of sodium hydroxide were added to 350 ml of 18.6 ppg brine. The solution was stirred for 2 days. To 35 ml of the sodium hydroxide treated brine, 0.20 grams of sodium benzoate were added and the brine was magnetically stirred. After an additional 2.5 days, 0.42 grams of sodium benzoate were added to the mixture. The mixture was stirred for an additional 2 days at which point the brine was filtered through fine DE. The initial iron content of 1802 mg / L (808 ppm) was reduced to 16 mg / L (7 ppm).
PUM
| Property | Measurement | Unit |
|---|---|---|
| densities | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| resting time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com