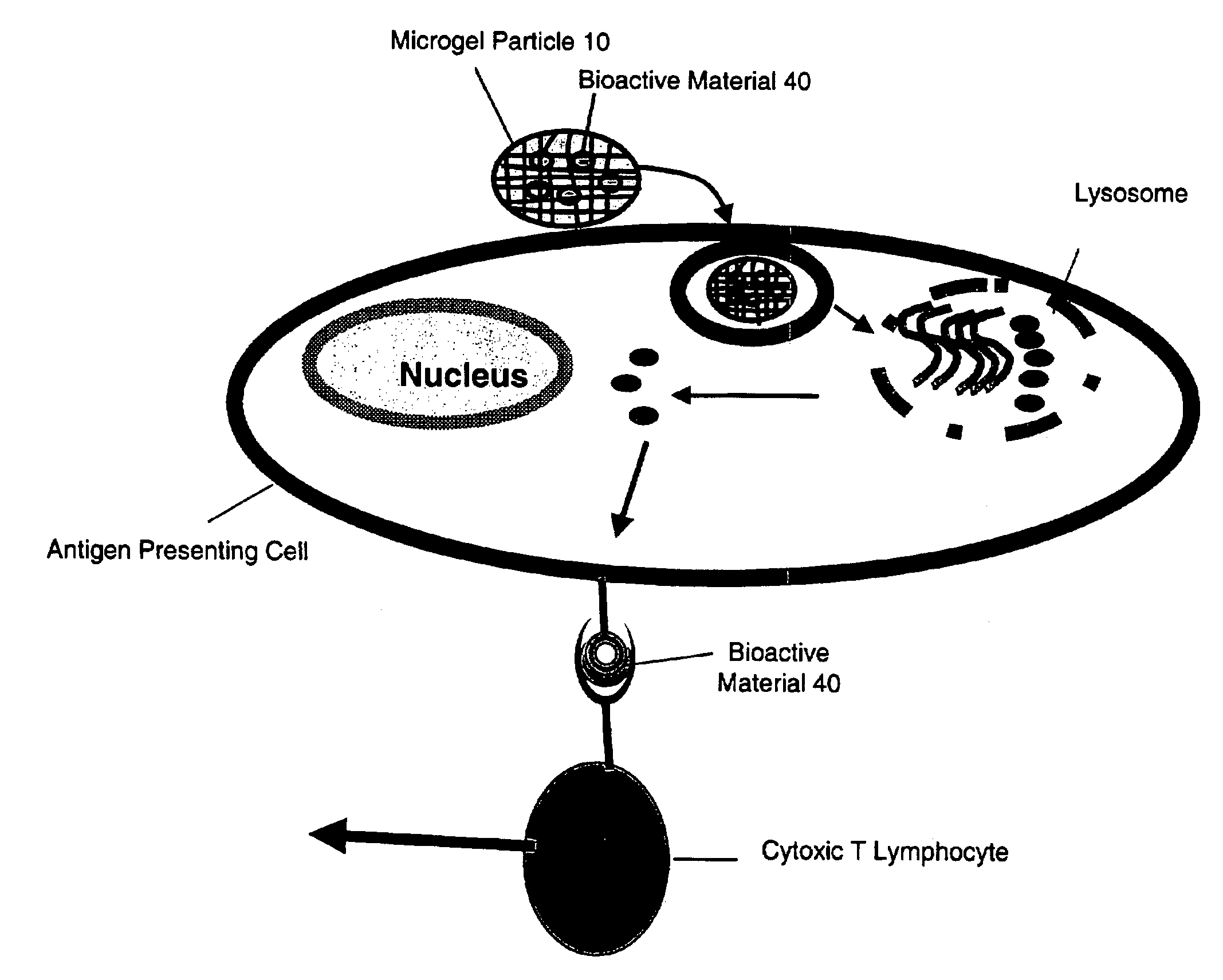
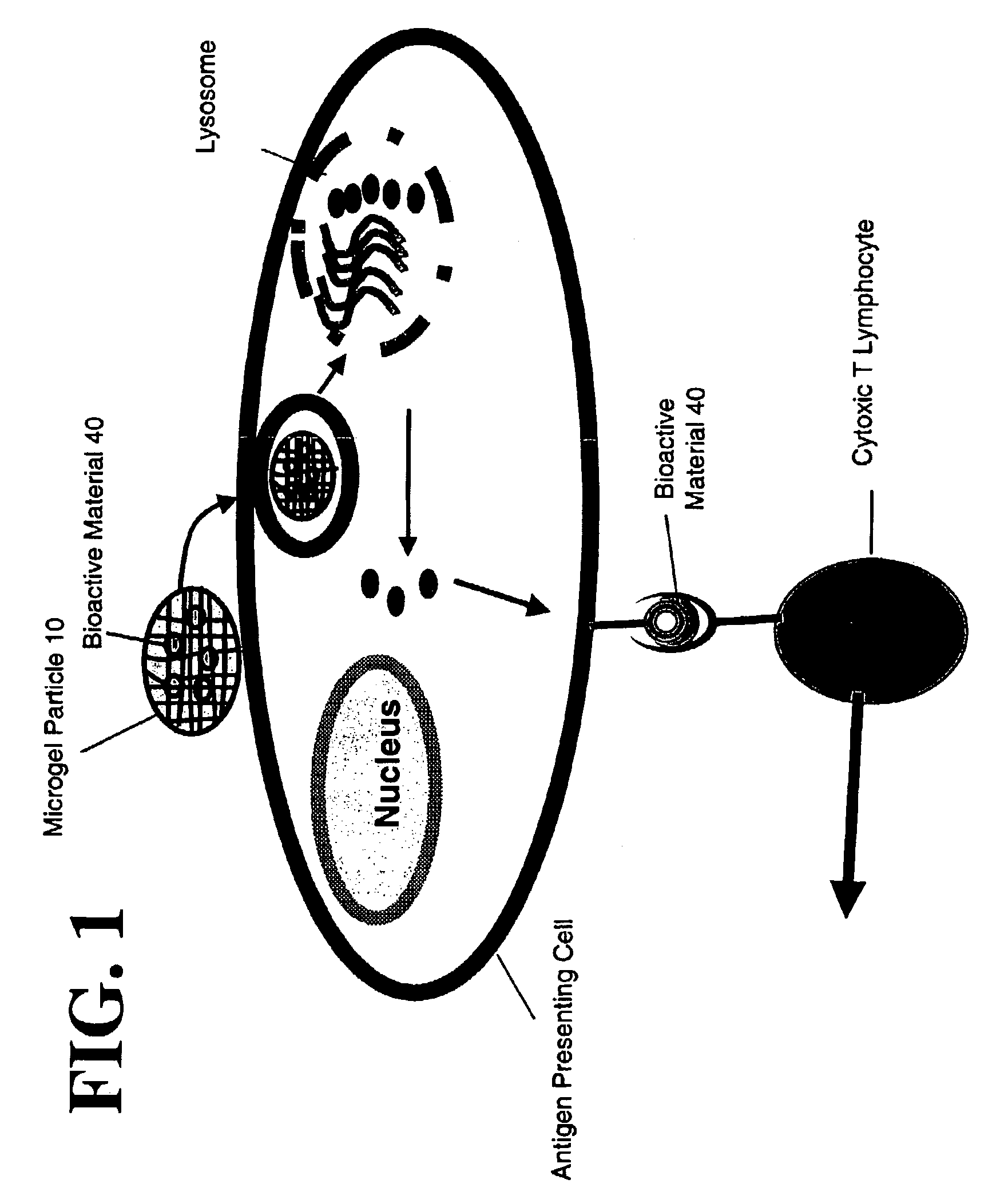
Microgel particles for the delivery of bioactive materials
a bioactive material and microgel technology, applied in the direction of microcapsules, powder delivery, peptides, etc., can solve the problems of protein-based vaccines, ineffective vaccine generation strategies based on live attenuated viruses, and limited clinical success of protein-based vaccines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis and Delivery of Microgels
[0137]Referring now to FIG. 3, an exemplary process for the synthesis of a microgel particle for pH-dependent degradation is shown. A polymerizable acrylic group 100 is added to the bisacrylamide methoxybenzaldehyde acetal crosslinker 110 (which is described in the following example) wherein R1=(a) of FIG. 2 and R2=aryl-methoxy. The polymerization reaction is carried out by inverse microemulsion, initiated by an initiator such as TMEDA and diammonium persulfate and carried out in the presence of bioactive particle 40, and carried out in the presence of bioactive particle 40.
[0138]At neutral pHs, the bisacrylamide methoxybenzaldehyde acetal crosslinker 110 remains intact and the release of entrapped bioactive material 40 is significantly slower or not at all. Under acidic conditions, the acetal group hydrolyses and increases the pore size of microgels made with it, releasing the entrapped bioactive materials 40, and degraded particles, in the form o...
example 2
Synthesis of a Bisacrylamide Methoxy Benzaldehyde Acetal Crosslinker
[0139]Referring to FIG. 4, the bisacrylamide methoxy benzaldehyde acetal crosslinker 110 was synthesized in two steps. The first step was acetal formation with hydroxy-trifluoroacetamide and methoxy benzaldehyde using the procedure of Roelofsen et al., Recueil, 90, 1141–1152 (1971, which gave a 70% yield of bistrifluoroacetamide methoxy benzaldehyde acetal 410 after chromatography. In the second step, the intermediate was then deprotected with 6N NaOH and reacted with acryloyl chloride to yield the crosslinker 110 (FIG. 3) in 40% yield after chromatography. In the crosslinker 110, R2 (according to the generic formula of FIG. 2) was methoxy benzaldehyde (methoxy aryl).
example 3
Synthesis of an Autocatalytic Bisacryloyl Acetal Crosslinker
[0140]A strategy for synthesis of an autocatalytic bisacryloyl acetal crosslinker involves the condensation of one molecule of carbonyl compound such as a benzaldehyde with two molecules of a carboxylic acid or derivative thereof. In this case, R1=(c) of FIG. 2, and R2=aryl-H.
[0141]FIG. 5A shows a bisacrylic benzaldehyde acetal crosslinker 530 obtained by incorporation of two acrylic acid moieties in an acetal like structure with benzaldehyde. Referring now to FIG. 5A, para methoxy benzaldehyde 510 is reacted with acrylic anhydride 520 in the presence of sulfuric acid to form the autocatalytic bisacrylic benzaldehyde acetal crosslinker 530. Also shown is an alternative synthesis is to react dibromo-toluene with sodium acrylate 550 to form the crosslinker 530.
[0142]In addition to the same acid degradable linkage, hydrolysis of the autocatalytic crosslinker under mild acidic conditions proceeds with release of two molecules o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com